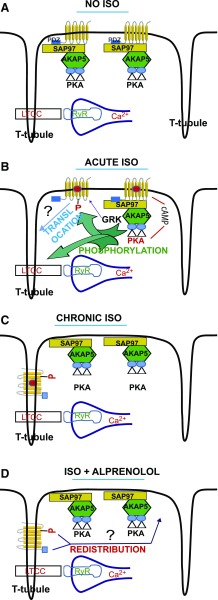
Fig. 6.
Effect of ISO on WT β1-AR compartmentalization in ARVMs. (A) In naive no ISO ARVMs, WT β1-AR are confined to the surface plasmalemmal membrane. In this compartment, the β1-AR associates with the PDZ protein SAP97 via its type-1 PDZ. SAP97 in turn binds to the AKAP5/PKA (Gardner et al., 2007) complex. (B) Exposing ARVMs to ISO activated the β1-AR signaling pathway and increased cAMP, which in turn activated PKA bound to the β1-AR microdomain. The activated catalytic subunits of PKA phosphorylate many proteins including the β1-AR on Ser312, L-type calcium channels (LTCCs) and many others. In conditions associated with chronic ISO, the agonist-activated β1-AR translocated away from the SAP97/AKAP5/PKA complex on the surface membrane to punctate invaginations that appear to correspond to T-tubules by an, as yet, undetermined mechanism. (C) Our results show that under chronic ISO conditions, the β1-AR resides exclusively in these intracellular structures that overlap with the T-tubule-specific dye Di-8 ANEPPS. (D) Upon replacing ISO with the β-blocker ALP, internal β1-AR recycled in a type-1 PDZ- and phospho-Ser312-dependent manner from their intracellular compartment to the surface plasmalemmal membrane to restore the organization depicted in the no ISO condition shown in (A). RyR, Ryanodine receptors.