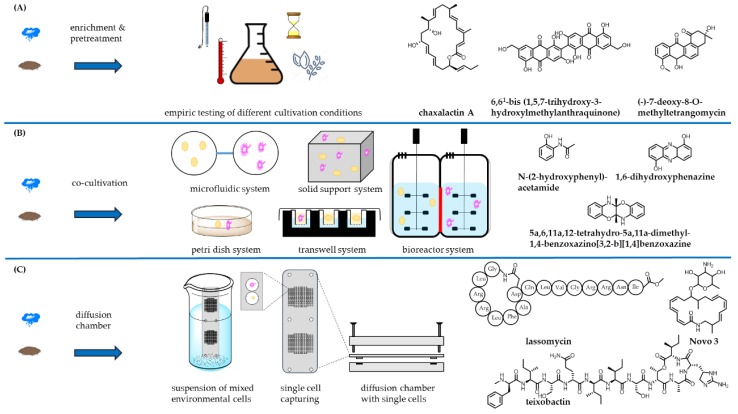
Figure 1.
Scheme of isolation strategies, partly adapted from Nichols et al. [33] and Goers et al. [39]. (A) Soil sample or marine sample undergoes enrichment and/or pretreatment to increase the chance to isolate new species and/or reduce undesirable background from previously isolated strains. In general, empirical established methods for fermentation varies in incubation time, media composition, additives, pH and temperature to enable growth of desirable strains. Chaxalactin A produced by Streptomyces sp. C34 [26,27], 6,61-bis (1,5,7-trihydroxy-3-hydroxylmethylanthraquinone) produced by Streptomyces spp. ERI-26 [29,30] and (-)-7-deoxy-8-O-methyltetrangomycin from Nocardiopsis sp. HR-4 [28] are examples for novel metabolites found using this conventional method. (B) Soil sample or marine sample is co-cultivated with other microorganisms to promote culturable isolates or to stimulate the secondary metabolism. Co-cultivation is categorised in microfluidic systems, petri dish co-culture systems, co-cultures on solid supports, co-culture systems using bioreactors and transwell systems [39]. Co-cultivation of Actinokineospora sp. EG49 and Nocardiopsis sp. RV163 induces the biosynthesis of three natural products namely N-(2-hydroxyphenyl)-acetamide, 1,6-dihydroxyphenazine and 5a,6,11a,12-tetrahydro-5a,11a-dimethyl[1,4]benzoxazino[3,2-b][1,4]benzoxazine [40]. (C) Soil sample or marine sample is used to create a suspension of mixed environmental cells. The isolation chip (iChip) plate is immersed into this suspension to capture (on average) a single cell. Covered with an upper and lower plate, the assembled iChip provides a miniature diffusion chamber for each single cell [33]. NOVO 3 [36], the cyclic peptide lassomycin [37] and the antibiotic teixobactin [38] were isolated from strains obtained by the iChip technology.