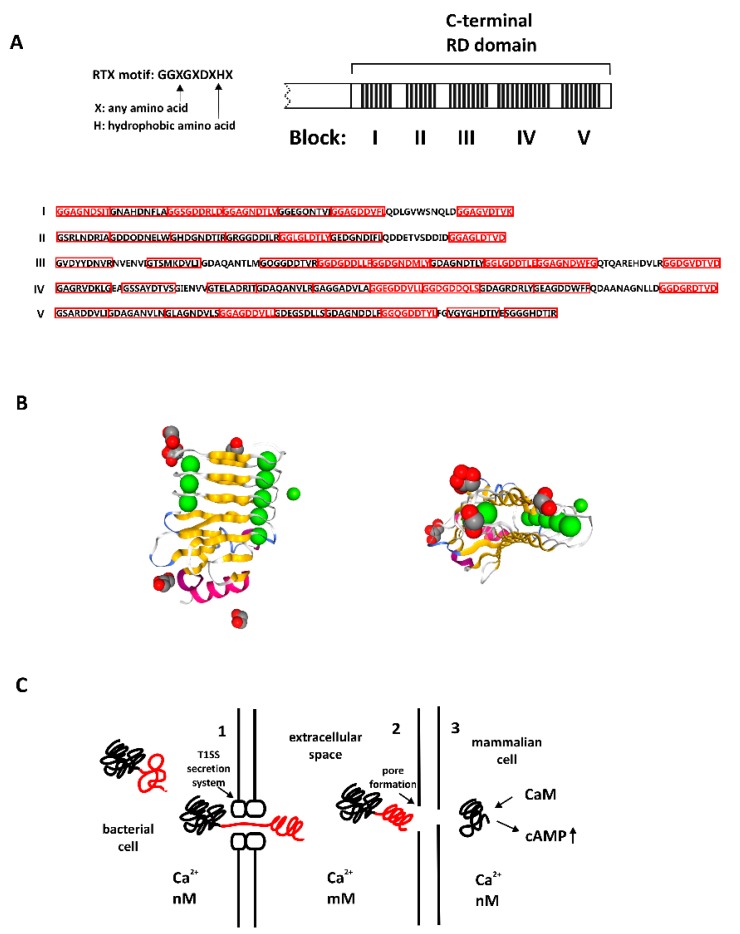
Figure 1.
Structure and function of bacterial adenylate cyclase toxin (CyaA). (A) Structure of the C-terminal RTX domain (RD) of CyaA, with five blocks containing repeat-in-toxin (RTX) consensus. Variability of the motif can be observed in all blocks, RTX motifs in red frames, full consensus in red letters. (B) Left panel: calcium-bound RTX domain of bacterial toxin CyaA, β-roll structure, PDB_5CVW, right panel: the same structure from another perspective, visible narrow parallel β-roll. (C) Bacterial toxin with RTX domain acquires the structure upon Ca2+ binding. 1. Protein in bacterial cell: C-terminal domain is unstructured (red) in a low Ca2+ concentration. 2. Protein translocates into extracellular space through type 1 secretion system (T1SS): in a high concentration of calcium ions C-terminal domain acquires secondary structure (β-roll) 3. The toxin forms pores in mammalian cell membrane; part of the toxin (catalytic domain) translocates into the mammalian cell, binds calmodulin and causes detrimental increase in cellular cAMP.