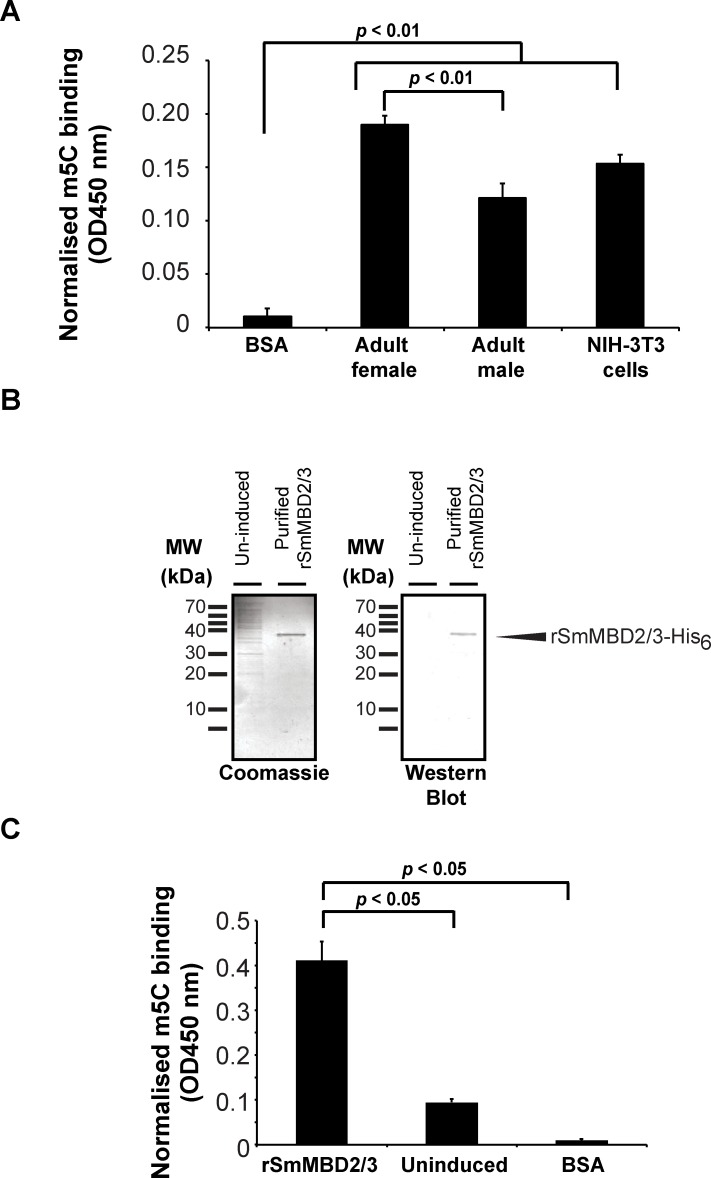
Fig 3. Schistosome nuclear protein extracts and recombinant SmMBD2/3 both contain 5mC binding activities.
(A) The 5mC binding capacity of nuclear protein extracts derived from adult male and female worms were quantified using the Epigentek MBD2 binding activity/inhibition assay. NIH-3T3 nuclear protein extracts and BSA were included as positive and negative controls, respectively. A significant difference in 5mC binding (in the CpG context) amongst protein samples was found. (B) IPTG-induced rSmMBD2/3-His6 protein (arrowhead; 36.5 kDa) was produced in E. coli, purified by Ni2+-NTA column chromatography and subjected to MALDI-TOF MS (Materials and Methods; 22 peptides covering 67% of full length SmMBD2/3 identified). An un-induced sample was also produced and similarly processed. (C) The 5mC binding activity (within a CpG context) of purified rSmMBD2/3-His6 was measured using the Epigentek MBD2 binding activity/inhibition assay and compared to un-induced bacterial and BSA protein samples. Significant differences in 5mC binding were observed between rSmMBD2/3-His6 and both the BSA and un-induced samples.