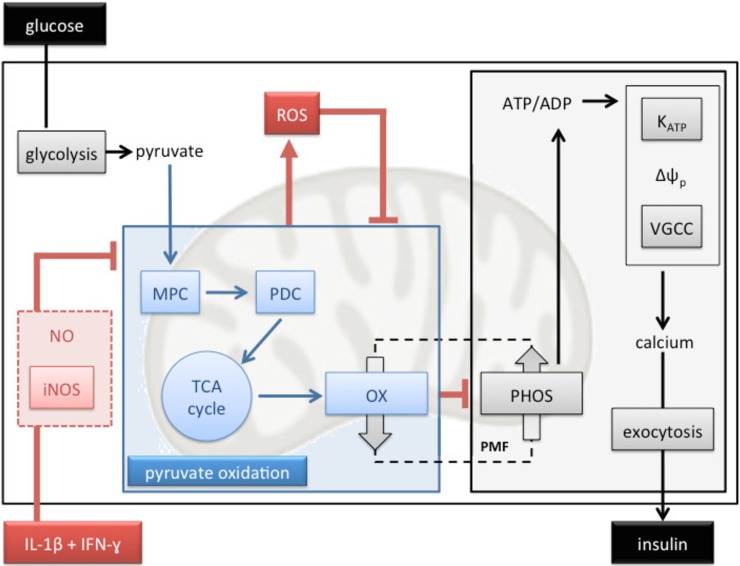
Fig 5. Mechanistic model of cytokine-induced GSIS impairment.
Pancreatic β-cells respond to a rise in the blood glucose level by increasing their oxidative metabolism. Pyruvate that results from increased glycolysis is imported to mitochondria by the mitochondrial pyruvate carrier (MPC) and then oxidized by the pyruvate dehydrogenase complex (PDC) to acetyl-CoA, which fuels the tricarboxylic acid (TCA) cycle. Reducing power generated by these processes is oxidized by the mitochondrial electron transfer chain (OX) that conserves liberated energy in a protonmotive force (PMF), which drives ADP phosphorylation (PHOS). The consequent rise in the cytosolic ATP/ADP ratio inhibits ATP-sensitive potassium channels (KATP), decreases the plasma membrane potential (Δψp), opens voltage-gated calcium channels (VGCC), causes Ca2+ influx, and triggers exocytosis of insulin-containing granules. Exposure to IL-1β plus IFN-γ inhibits mitochondrial pyruvate oxidation (blue box) likely via activation of inducible nitric oxide synthase (iNOS) and consequent NO formation. Inhibition of pyruvate oxidation dampens bioenergetic glucose sensitivity and thus attenuates GSIS. Cytokine-induced reactive oxygen species (ROS) are a plausible consequence of inhibited pyruvate oxidation and may reinforce this inhibition.