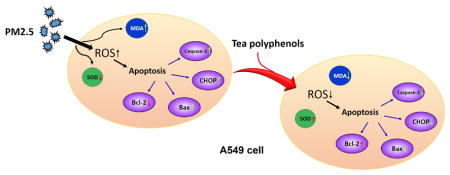
Abstract
Tea-derived polyphenols have anticancer and antioxidant properties, and they can regulate oxidative stress. This study was designed to quantify both the toxic effects of fine particulate matter with aerodynamic diameter less than 2.5 μm (PM2.5) and determine whether tea polyphenols could provide a protective effect against PM2.5 toxicity on human alveolar epithelial A549 cells in vitro. Cytotoxic effects of the PM2.5 on A549 cells were measured by means of cell viability, the expression of caspase-3, bax/bcl-2 and C/EBP-homologous protein (CHOP), and the generation of intracellular reactive oxygen species, malondialdehyde and superoxide dismutase. The results showed that tea polyphenols ameliorated some of the adverse effects of PM2.5 on A549 cell viability and superoxide dismutase levels. In addition, tea polyphenols decreased the production of reactive oxygen species, malondialdehyde generation, and apoptosis in response to PM2.5 exposure. Therefore, our results support a role for tea polyphenols in reducing the toxicity of PM2.5, particularly with regard to targeting oxidative stress and apoptosis.
Keywords: Tea polyphenols, PM2.5, oxidative stress, apoptosis, lung cells
Graphical abstract
INTRODUCTION
PM2.5 is a major environmental pollutant that has become a significant threat to human health. The World Health Organization maintains an ongoing database identifying global exposure rates for fine particulate matter (PM2.5) and their data suggests that a majority of the human population that live in cities have been exposed to PM2.5 to varying degrees (http://www.who.int/gho/phe/air_pollution_pm25_concentrations/en/). Moreover, extensive epidemiological studies have identified PM2.5 exposure as a major variable associated with increased morbidity and mortality for cardiopulmonary and respiratory diseases, such as cerebrovascular disease, stroke, heart rhythm disturbances, chronic obstructive pulmonary disease, and respiratory tract infection (Leiva et al., 2013; Bell et al., 2014; Karlsson et al., 2005; Pardo et al., 2015). One reason for the danger associated with PM2.5 exposure is based on its size; with a diameter less than 2.5 μm, these particles are able to penetrate deeply into the alveolar tissue of the respiratory tract. The alveolar duct cells and adjacent capillary beds are, therefore, vulnerable to PM2.5 and this may constitute a critical route of physiological challenge associated with the exposure risk of PM2.5 and the subsequent adverse effects for human health (Bell et al; 2010; Guo et al., 2015; Loxham et al., 2015).
Current standard practice for protecting individuals against PM2.5 exposure have largely utilized barrier or filtration approaches to try and reduce particle inhalation. Outdoor interventions primarily involve reduced exposure time and/or the use of facial masks as a primary inhalant barrier while efforts to reduce indoor PM2.5 exposure largely rely on central air conditioner and airflow systems in buildings to filter out particles (Cai and He, 2016). One study demonstrated the potential of using polyacrylonitrile transparent materials associated with windows to increase filtration efficiency for particulate matter, including PM2.5 (Liu et al., 2015). Despite these precautions, inhalation of PM2.5 is often unavoidable, particularly during cold, seasonal weather (Hu et al., 2017; Tahri et al., 2017). If the PM2.5 is inhaled, there is the potential for injury to the lung cells, and possibly the associated capillaries (Xu et al., 2017).
The hazardous effects of PM2.5 particles are largely caused by its small size, but composition of the particles may play a role as well. Indeed, composition can vary depending on source location as well as temporal and regional variation (Villar-Vidal et al., 2014; Akhtar et al., 2014; Sun et al., 2014; Liang et al., 2017). Because of their small size and high surface area/volume ratio, PM2.5 have been shown to be able to penetrate into mucous-lined epithelial cells in culture, along with much smaller ultrafine particles (Loxham et al., 2015). Moreover, the PM2.5 often contain toxic components such as polycyclic aromatic hydrocarbons (PAHs; Oh et al., 2011) and transition metals (Al, Pb, Cu, etc.; Rodríguez-Cotto et al., 2014) that increase the potential toxicity of PM2.5. One of the primary mechanisms whereby PM2.5 appears to mediate cellular toxicity is via its ability to increase oxidative stress in a cell or tissue system. Oxidative stress is one indicator of current or predictive tissue damage and oxidative stress is generally characterized by an increase in the levels of reactive oxygen species (ROS) and a concomitant reduction in the activity of superoxide dismutase (SOD; Cachon et al., 2014). Therefore, this research focuses on examining a potential treatment method, tea polyphenols, and their ability to ameliorate the toxic effects of PM2.5 exposure.
Polyphenols derived from tea extracts have been characterized for their potential health benefits in managing oxidative stress (reviewed in Mao, 2017; Yiannakopoulou, 2013; Saeed et al., 2017; Malongane et al., 2017) and for their ameliorating effects in a variety of human diseases and animal models of human diseases (Paola et al., 2005; Xie et al., 2012; Lorenz, 2013; Renno et al., 2017). The current study focuses on the antioxidant capacity of tea polyphenols to treat or prevent apoptosis caused by PM2.5 exposure in a lung culture model. Herein, we quantified changes in two toxicity metrics, oxidative stress indicators and apoptosis, and quantified the ability of tea polyphenols to ameliorate the damage induced by PM2.5 exposure in lung cells.
MATERIALS AND METHODS
Preparation of PM2.5
PM2.5 samples from Shijiazhuang city were collected in an area known for heavy vehicular traffic with a high exposure to diesel exhaust. PM2.5 samples were collected on glass filters (diameter = 90 mm, Whatman, USA) using a high volume sampler (average collection rate at 24 L/min, Beijing Geology Device Company, China) for 12 hours (8:00–20:00). The glass filters were preheated at 500 °C for 4 hours before sampling, and weighed on a microbalance (Mettler Toledo, USA) to measure atmospheric daily PM2.5 concentration collected. All sample filters were stored in the dark at −20 °C before further chemical and physical characterization to avoid light-based sample degradation or contamination. Unexposed filters were used as a control and were prepared in parallel using the same methods. The PM2.5 samples were extracted from sample filter strips or control strips by immersing them in methanol:deionized water (v: v=4:1) and sonicating for 60 min at room temperature. The sonicated methanol:water mixture constituted the extracted material and these samples were then incubated at 65 °C for 12 hours to dry the material. The dried samples were suspended in serum free culture medium containing 0.1% DMSO and stored at 4 °C.
Cell Culture and Treatment
Human lung carcinoma cell line A549 (ATCC CCL-185) was grown and maintained in Dulbecco’s modified Eagle medium (DMEM, Sigma–Aldrich, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 2 mmol/L glutamine, and 100 IU/mL penicillin, 100 μg/mL streptomycin. During the whole process, growing cells were cultured at 37°C, in a humidified environment containing 5% CO2. The A549 cells were seeded onto 60 mm tissue culture dishes and allowed to adhere for 18 hours. The media was removed and replaced with either control media or media containing PM2.5 for 24 hours. After the PM2.5 was removed, tea polyphenol was added to the media for 24 hours. Tea polyphenols were purchased as powder and added at the defined concentrations indicated after solubilization in water (Shanghai Jonln Reagent Company, Shanghai, China).
Cell Viability Assay
The effect of PM2.5 and tea polyphenols on cell viability was measured using the Cell Counting Assay Kit 8 (CCK-8, Yeasen, China) according to the manufacturer’s protocol. A549 cells were seeded at a density of 2×103 cells/well into 96-well flat-bottomed plates and allowed to adhere overnight (18 hours). Cells were then exposed to 0.1 – 500 μg/mL of PM2.5 for 24 hours and 0.1 – 100 μg/mL of tea polyphenols for 24 hours, in series. At the end of the assay time period, 10 μL of CCK-8 developing solution was added to each well and incubated for 2 hours at 37°C. The proportion of viable cells was determined as a function of absorption at 450 nm (Bio-Tek Microplate Reader, USA).
Measurement of Intracellular ROS level, SOD activity and MDA Levels
The assays to measure ROS, SOD, and MDA were carried out by reactive oxygen species assay kit, total superoxide dismutase (T-SOD) assay kit and catalase (cat) assay kit (Jian Cheng Bioengineering Institute, Nanjing, China), respectively, according to the manufacturers’ protocols. The assays were set up as described for the viability assay above. At the end of the assay period, the cells were washed with phosphate buffered saline solution (PBS) and incubated with 10 μmol/L 2′,7′-dichlorofluorescin diacetate (DCFH-DA) in DMEM medium in a CO2 incubator at 37°C for 30 minutes. The DCFH-DA is de-esterified intracellularly and produces a measurable green fluorescent oxidation product in the presence of ROS. After the 30-minute incubation, the cells were washed again with PBS to remove excess DCFH-DA and 1.0 mL of PBS was added to each well. The fluorescence intensity as a reflection of intracellular ROS levels was quantified using a FACS Calibur flow cytometer (BD Biosciences, CA, USA), with an excitation wavelength set at 488 nm and an emission wavelength of 525 nm. Similar assay approaches were used to quantify SOD activity and the level of MDA product, using absorbance wavelengths set at 550 nm 532 nm, respectively, measured on a standard microplate reader (Bio-Tek, USA).
Apoptosis Detection Assay
The levels of apoptotic cells in the different treatment conditions were determined using double labeling for Annexin V and propidium iodide (PI) (Annexin V/PI assay kit, BD bioscience, US) and detection with flow cytometry according to the manufacturer’s protocol. The A549 cells plated at a density of 1 × 105 cells/well in 6-well plates and then challenged with 0.1, 1 and 10 μg/mL PM2.5 followed by tea polyphenol treatment as described previously. At the end of the assay, the cells were harvested, washed with cold PBS, and incubated in the dark with 1× Annexin V working solution containing PI (1 μg/mL final concentration) for 5 min at room temperature. The cells were exposed to binding buffer according to the manufacturer’s recommended protocol (400 μL of 1× binding buffer and sample cells). The proportion of apoptotic cells was determined using CellQuest software on a flow cytometer with gates set at default sensitivity to determine single and double-labeled populations. The cell number was set at 2×104 per test.
Western Blot Analysis for Cleaved Caspase-3, Bax/Bcl-2 and CHOP
The A549 cells were plated in 6-well plates and then challenged with PM2.5 followed by tea polyphenol treatment, as described previously. At the end of the treatments, A549 cells were washed once with PBS and then lysed with radioimmunoprecipitation assay (RIPA) buffer containing 1% phenylmethylsulfonylfluoride (PMSF) for 5 minutes on ice. The whole-cell lysates were centrifuged at 12,000 rpm for 5 min at 4 °C, and the supernatants were collected. Protein concentrations were determined by bicinchoninic acid assay against a standard curve (BCA/Smith Assay, Jian Cheng Bioengineering Institute, China). Equal amounts of protein (10 μg) were separated by electrophoresis on 10% sodium dodecyl sulphate- (SDS-) polyacrylamide gels and transferred onto 0.22 μm polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with 5% (w/v) non-fat milk powder in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST) for 90 min to block nonspecific binding sites on the membrane. The PVDF membranes were then incubated overnight at 4 °C with diluted primary antibodies (caspase-3 antibody, bax antibody, bcl-2 antibody and CHOP antibody, Cell Signaling, US). After extensive washing with TBST, the membranes were incubated for 90 min at room temperature with the horseradish peroxidase conjugated anti-rabbit secondary antibodies (Cell Signaling, US). After being rewashed with TBST, the bands were developed by enhanced chemi-luminescence detection reagents kit (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China).
Statistical analysis
The data were analyzed using a One Way ANOVA analysis (SPSS 21.0) with treatment condition as the dependent variable and the significance set at the 0.05 level. When both PM2.5 and tea polyphenol treatments were included in an assay, the results were analyzed with Two Way ANOVA, the post hoc test used was Tukey HST and the P values were provided in the figure legends and indicated on the graphs.
RESULTS
PM2.5 and Tea Polyphenols Cytotoxicity
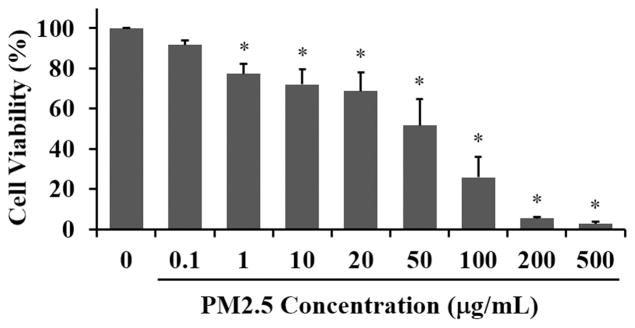
Since PM2.5 has considerable toxic impact based on the composition of the chemical components and the ability of the fine particulate matter to penetrate deep into the lung alveolar buds, we wanted to determine the adverse effects of PM2.5 on cultured human lung cells. We treated A549 human lung carcinoma cells with a range of PM2.5 doses from 0.1 to 500 μg/mL for 24 hours (Fig. 1). We observed statistically significant, concentration-dependent reduction in cell viability determined via the CCK-8 assay with the highest doses resulting in nearly 100% cell loss. Even at a concentration of 1 μg/mL PM2.5, cell viability was 77.25 ± 4.88 % relative to control cells. Based on these results we chose to use PM2.5 at a concentration of 50 μg/mL which resulted in ~50% cell viability. Our rationale for this concentration was to use a mid-point effective dose that would result in a damaged, but not a crashing cell population.
Fig. 1. Cell viability in A549 cells is reduced with PM2.5 exposure.
The human lung carcinoma cell line, A549, was exposed to PM2.5 (0 to 500 μg/mL) for 24 hours and viability detected using the Cell Counting Assay Kit (CCK-8). The percent viability is graphed and each bar represents the mean ± SD with n = 6 samples per condition. The asterisks indicate statistically significant differences between exposed and control cells (*) P< 0.05.
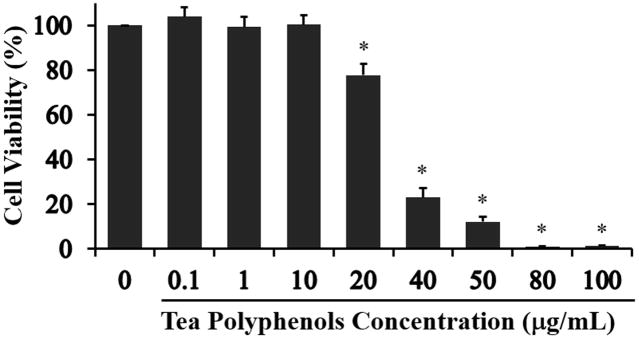
In order to investigate tea polyphenols as a potential therapeutic intervention for PM2.5-induced toxicity in lung cells, we first needed to identify a dose that would allow for an effective range without having a secondary detrimental impact on the cells. While tea polyphenols have been shown to be beneficial to human health and nutrition, some reports indicate adverse effects at high tea polyphenols concentrations in kidney and liver cells (Yang et al., 1998; Murakami, 2014; Inoue et al., 2011) with both anti-oxidant and pro-oxidant effects reported, depending on cell type and dose (reviewed in Lorenz et al., 2013). Therefore, we treated A549 cells with a range of tea polyphenol from 0.1 μg/mL to 100 μg/mL and then quantified cell viability in order to identify an optimal concentration for our assays (Fig. 2). Tea polyphenol concentrations at 20 μg/mL and higher significantly reduced cell viability with 100% cell loss observed at 80 μg/mL and 100 μg/mL. This result demonstrated that tea polyphenols have cytotoxicity at high concentrations in agreement with the results of others (Murakami, 2014; Inoue et al., 2011). Based on our cell viability assessment, we chose to use the non-toxic concentration of tea polyphenols in the range of 0.1 to 10 μg/mL for subsequent experiments.
Fig. 2. Concentration range impact of tea polyphenols on A549 cell viability.
A549 cells were exposed to tea polyphenols (0 to 100 μg/mL) for 24 hours and viability assessed using the CCK-8 assay. The percent viability is graphed and each bar represents the mean ± SD with n = 6 samples per condition. The asterisks indicate statistically significant differences between exposed and control cells (*) P< 0.05.
Tea Polyphenols Reduce Oxidative Stress Induced by PM2.5 in Lung Cells
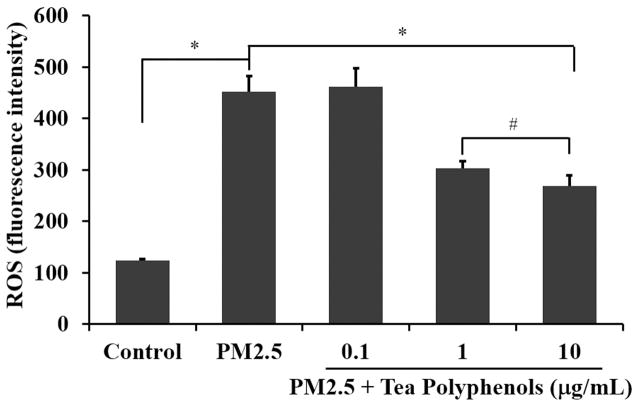
Since one of the proposed mechanisms of action for PM2.5 toxicity is via oxidative stress, we anticipated that PM2.5 treatment could stimulate an oxidative stress response in the A549 lung carcinoma cells. We therefore chose to quantify levels of ROS, SOD and MDA as biological metrics of oxidative stress in A549 cells treated with PM2.5. Tea polyphenols have previously been demonstrated to reduce ROS in rat aortic endothelial cells fed on a high fat diet (Zuo et al., 2014). We postulated that a similar protective effect might occur in the A549 cells stressed with PM2.5 treatment. We quantified ROS levels in A549 cells treated with 50 μg/mL PM2.5 for 24 hours and then subsequently treated with 0.1 to 10 μg/mL of tea polyphenols (Fig. 3). PM2.5 induced an increase of ROS in comparison with the control group (shown in Fig. 3, P<0.01). Treatment with 1 μg/mL or 10 μg/mL tea polyphenols reduced ROS levels compared to A549 cells treated with PM2.5 alone (Fig. 3 P<0.01). The ROS levels in the samples treated with tea polyphenols did not reach the levels of ROS in the control, untreated population. However, obviously, ROS level of tea polyphenols treatment groups was lower than only PM2.5 treatment group, and the treatment effect of 10 μg/mL tea polyphenols was better than 1 μg/mL.
Fig. 3. Tea polyphenol treatment attenuates ROS levels in PM2.5-treated A549 cells.
The A549 cells were exposed to 50 μg/mL PM2.5 for 24 hours and then treated with tea polyphenols (0.1 to 10 μg/mL) for an additional 24 hours. The ROS levels, expressed as fluorescence intensity, are graphed as the mean ± SD of n=6 samples per condition. The asterisks indicate statistically different conditions with post hoc pairwise comparisons indicated with connecting lines for (#) P< 0.05, (*) P< 0.01.
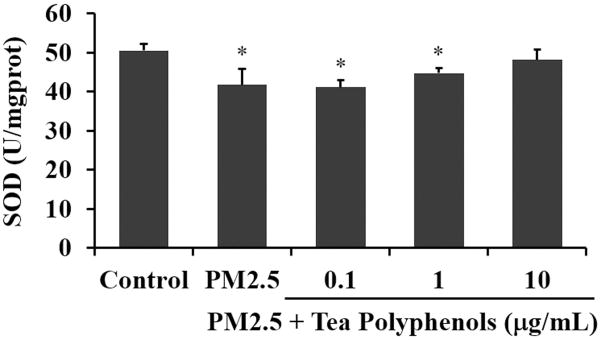
The activity levels of SOD in a healthy cell are maintained to regulate superoxide production downstream of oxygen metabolism (Griess et al., 2017). We quantified SOD activity levels in A549 cells treated as described for Figure 3 and determined that SOD activity levels were significantly decreased after exposure to PM2.5 for 24 hours (Fig. 4). However, after treatment with tea polyphenols for 24 hours, the SOD activity levels were increased at the higher doses with recovery of control levels observed at 10 μg/mL of tea polyphenols (shown in Fig. 4, P>0.05).
Fig. 4. Tea polyphenol treatment attenuates SOD activity levels in PM2.5-treated A549 cells.
The A549 cells were exposed to 50 μg/mL of PM2.5 for 24 hours and then treated with tea polyphenols (0.1 to 10 μg/mL) for an additional 24 hours. The SOD activity levels are expressed as units (U) of enzyme activity compared to mg protein added to the assay (mg prot) and graphed as the mean ± SD of n=6 samples per condition. Asterisks indicate statistically significant differences between exposed or treated and control groups (*) P< 0.05.
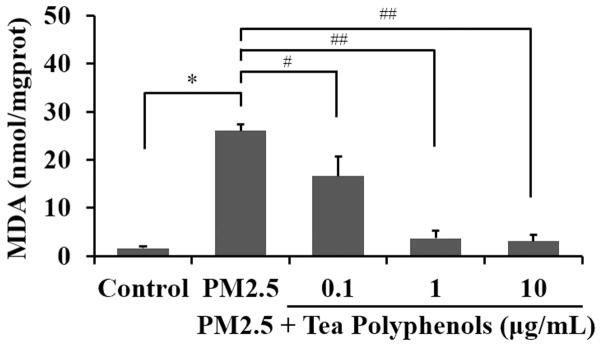
MDA is one of the primary byproducts of lipid oxidation and its production can be used as a biomarker of oxidative stress (Czerska et al., 2015). We quantified MDA levels in our PM2.5/tea polyphenol treatment paradigm and observed significantly elevated MDA levels in A549 cells treated with PM2.5 compared to the control group (Fig. 5, P<0.001). However, treatment with tea polyphenols after PM2.5 exposure ameliorated the MDA elevation with levels returning to ~90% of control at the 1 and 10 nmol/mg protein doses.
Fig. 5. Tea polyphenol treatment reduces MDA levels in PM2.5-treated A549 cells.
The A549 cells were exposed to 50 μg/mL of PM2.5 for 24 hours and then treated with tea polyphenols (0.1 to 10 μg/mL) for an additional 24 hours. The MDA levels are expressed as nanomoles of substrate (nmol) converted to reaction product relative to mg protein added to the assay (mg prot) and graphed as the mean ± SD of n=6 samples per condition. The asterisks indicate statistically different conditions with post hoc pairwise comparisons indicated with connecting lines for (#) P< 0.05, (##) P< 0.01 and (*) P< 0.001.
Tea Polyphenols Reduce Cell Apoptosis Level after PM2.5 treatment
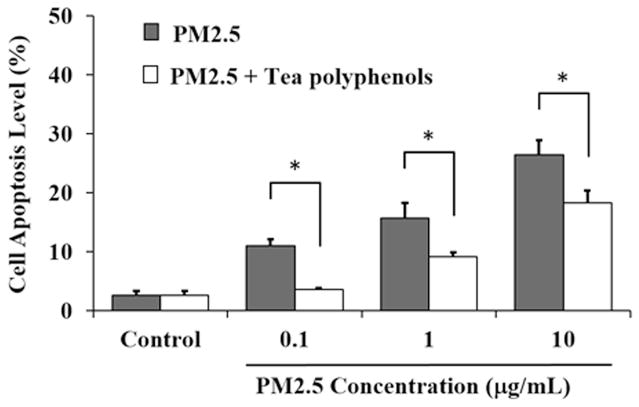
One of the hallmarks of PM2.5 toxicity includes the feature that these particles are able to directly impact cells and induce oxidative stress resulting in apoptosis. (Deng et al., 2013). We wanted to determine whether the tea polyphenols would have a protective effect against PM2.5-induced apoptosis in A549 cells. To that end, we quantified apoptosis levels in our PM2.5 toxicity lung cell model using colabeling for Annexin V/PI (Fig. 6). Based on ROS, SOD activity and MDA quantity level assay, 10 μg/mL of tea polyphenols had effective therapeutic effect as a treatment concentration. The tea polyphenols reduced the apoptotic effect of the PM2.5 significantly at each dose tested and the cell apoptosis quantified was dependent on the tea polyphenol concentration (Fig. 6). PM2.5 at 0.1, 1 and 10 μg/mL induced cell apoptosis up to 10.98 ± 1.13%, 15.76 ± 2.6 %, and 26.50 ± 2.37%, respectively. In contrast, treatment with tea polyphenols reduced the percentage of apoptotic cells to 3.55 ± 0.32%, 9.19 ± 0.68 %, and 18.25 ± 2.20%, respectively. These results support a role for the tea polyphenols in reducing the apoptosis of PM2.5-treated lung cells.
Fig. 6. Tea polyphenol treatment reduces apoptosis in PM2.5-treated A549 cells.
The A549 cells were exposed to 0.1, 1 and 10 μg/mL of PM2.5 for 24 hours and then treated with 10 μg/mL tea polyphenols for an additional 24 hours. Flow cytometry was used to analyze cell apoptosis. CellQuest software calculated the percentage of apoptotic cells. The cell apoptosis levels are expressed as percent relative to control and graphed as the mean ± SD of n=6 samples per treatment condition. The asterisks indicate statistically different conditions with pairwise comparisons indicated with connecting lines for (*) P< 0.05.
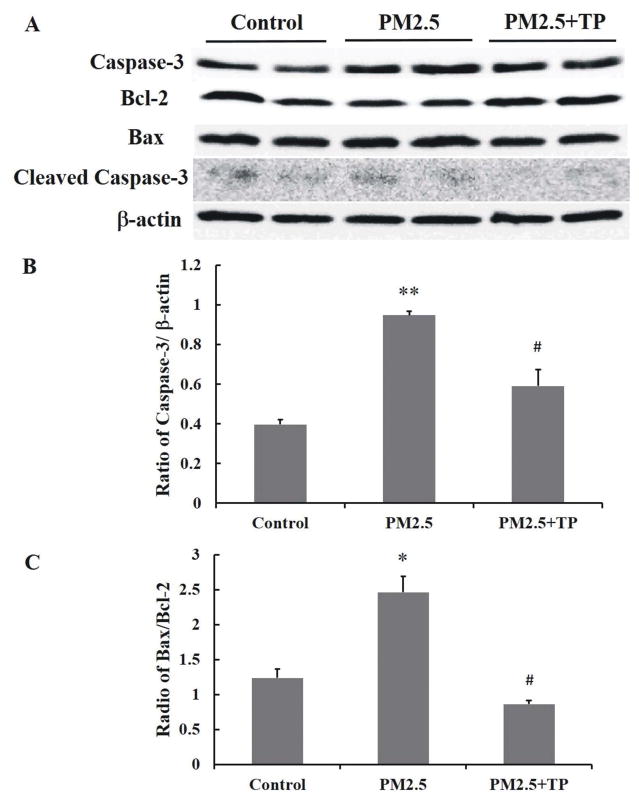
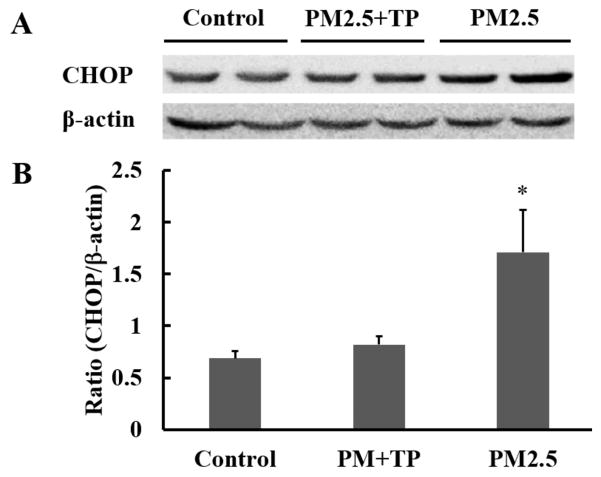
To further explore the potential mechanism for the protective effect of tea polyphenols on PM2.5-induced apoptosis, we wanted to determine protein levels of caspase-3, bax, bcl-2, and CHOP as key indicators of apoptosis (Cao et al., 2015; Liu et al., 2017). We quantified the bax/bcl-2 ratio and the caspase-3 expression levels in A549 cells treated with PM2.5 alone or PM2.5 followed by tea polyphenol treatment (Fig. 7). PM2.5 induced a significant increase in Caspase 3 and in the ratio of bax/bcl-2 compared with the control groups (P<0.05). However, with tea polyphenol treatment, the protein levels of caspase 3 (Fig. 7B) and bax decreased (P<0.05) while that of bcl-2 was increased (P<0.05) compared to the control group. As a result, the ratio of bax/bcl-2 dropped to control levels in the presence of tea polyphenols (Fig. 7C). CHOP protein levels also increased with PM2.5 treatment (Fig. 8) with CHOP levels reduced to control in the A549 cells that were also treated with tea polyphenols. Taken together our results indicate that PM2.5-induced cytotoxicity as measured by cell viability, oxidative stress assessment, and apoptosis in A549 lung cells was ameliorated by treatment with tea polyphenols.
Fig. 7. Tea polyphenol treatment attenuates PM2.5 treatment-induced increase in cleaved Caspase-3 levels in A549 cells.
(A) Western blot analysis of total cell lysate was performed on A549 cells exposed to 50 μg/mL PM2.5 for 24 hours and then treated with 10 μg/mL tea polyphenols (TP) for an additional 24 hours. β-actin was used as a loading reference to normalize across samples and treatment conditions. The density of the caspase-3, bcl-2, bax, and β-actin protein bands was quantified using Image Pro Plus software and the data graphed as the mean ratio ± S.E of cleaved caspase-3 relative to β-actin (B) and bax relative to bcl-2 (C) from three independent experiments with a total n = 3. Asterisks indicate statistically significant differences between exposed and control cells, (*) P< 0.05, (**) P< 0.01, and between exposed cells in PM2.5 and treated cells in tea polyphenols (#) P< 0.05.
Fig. 8. Tea polyphenol treatment attenuates PM2.5 treatment-induced increase in CHOP levels in A549 cells.
Western blot analysis of total cell lysate was performed on A549 cells exposed to 50 μg/mL PM2.5 for 24 hours and then treated with 10 μg/mL tea polyphenols (TP) for an additional 24 hours. Densitometric analysis of CHOP and β-actin bands was determined and the results expressed as the mean ratio ± S.E. of CHOP to β-actin for n=3 samples. The asterisk indicates statistical difference between control and PM2.5-treated populations, (*) P< 0.05.
DISCUSSION
PM2.5 are a major component of urban pollution and the complex chemical components and particle size of PM2.5 can directly aggravate the respiratory system and cause disease (Leiva et al., 2013; Bell et al., 2014; Karlsson et al., 2005; Pardo et al., 2015). The primary route of exposure is via PM2.5 inhalation into the lungs, resulting in tissue damage or distress that can disrupt normal lung function. Therefore, reducing or preventing inhalation, at the outset, in combination with reducing the end point toxic effects of PM2.5 are important goals with regard to human health and exposure to small particle pollutants. The tea polyphenols have been reported to be effective scavengers of reactive oxygen and nitrogen species and may help keep antioxidant enzyme activities in check in vivo (reviewed in Kim et al., 2000; Frei and Higdon, 2013; Raza and John, 2008). In the current study, we investigated the impact of tea polyphenols on alleviating the cytotoxicity of PM2.5 in a lung cell culture model. Using a variety of methods to assess cell survival and oxidative stress, we have obtained results indicating that tea polyphenols reduce the overall toxicity of PM2.5 on A549 cells.
PM2.5 exposure and increased oxidative stress have been associated in a variety of model systems, including rat cardiac cells that show increased ROS and subsequent activation of MAP kinase pathways in response to PM2.5 treatment (Cao et al., 2015). In addition, human lung epithelial cells exposed to PM2.5 in culture showed a marked increase in different apoptosis pathways, including increased caspase-3 cleavage, a shift in the ratio of bax to bcl-2 proteins in L132 cells (Dagher et al., 2006), and autophagy in A549 cells (Deng et al., 2013). Given the adverse consequences of PM2.5 on lung cells, it is important to identify treatment paradigms that can help prevent damage after fine particle exposure. In our study, we focused on the expression of caspase-3, bcl-2, bax and CHOP as a reflection of apoptosis. Bcl-2 and bax family members regulate mitochondrial outer membrane permeabilization (MOMP) to regulate apoptosis (reviewed in Kalkavan and Green, 2017). The expression levels of bcl-2 and bax in our model system suggested that the A549 cells responded to PM2.5 with increased apoptosis that might have been mediated, in part, by the mitochondrial apoptosis pathway. Moreover, levels of CHOP protein have been shown to play an important role in the response of the endoplasmic reticulum to stress (Gorman et al., 2012; Li et al., 2014; Liu et al., 2016). The elevation of CHOP linked to PM2.5 exposure was also alleviated by tea polyphenol treatment, indicating that stress effects mediated via the endoplasmic reticulum response pathway may be another target for reducing cell death.
Taken together, these results demonstrated that tea polyphenols at low concentrations could significantly inhibit oxidative stress and apoptosis induced by PM2.5. The green tea polyphenols contain catechin derivatives such epigallocatechin-3-gallate (EGCG; Renno et al., 2017); theaflavins (Leung et al., 2001) and thearubigins (Menet et al., 2004). Tea polyphenols, especially the derivative epigallocatechin gallate, are currently being explored in a variety of clinical and pre-clinical studies targeting neuropathic pain and cancer (reviewed in Bimonte et al., 2017; Fujiki et al., 2017; Sur et al., 2017; Lernoux et al., 2017). Previous studies have demonstrated that tea polyphenols are able to protect fibroblasts from peroxide-induced damage (Jie et al., 2006) and they can regulate oxidative stress by scavenging free radicals, chelating transition metal ions, and decreasing the accumulation of intracellular ROS (reviewed in Frei and Higdon, 2003; Raza et al., 2008). Moreover, the green tea catechin, EGCG, reduced the apoptosis of a human lung cancer line (PC-9) in combination with a synthetic retinoid, although oxidative stress was not assessed in that particular study (Oya et al., 2017). However, an investigation directed at determining tea polyphenols’ effect on mitigating particulate matter toxicity, such as with PM2.5-induced toxicity, has not been reported in lung cells and our research sought to fill this gap in knowledge. Our data support an ameliorative effect of tea polyphenols on regulation of oxidative stress markers (ROS level, SOD activity and MDA level) and apoptosis proteins (caspase-3, bcl-2, bax and CHOP). In conclusion, we present here a novel application for tea polyphenols in reducing the cytotoxicity induced by PM2.5 in lung cells. Tea polyphenols can be extracted from either green tea or black tea (Lee et al., 2017; Seo et al., 2016) and its constituents have been carefully defined (Sang et al., 2011). We suggest that tea polyphenols should be further explored as a cost-effective and accessible method to help alleviate long-term damage to lung cells associated with exposure to PM2.5.
PRACTICAL APPLICATIONS.
PM2.5 poses an ongoing threat to human health as a significant environmental pollutant, regionally and globally. We sought to test the ability of a known anti-oxidant, tea polyphenols, for their ability to ameliorate PM2.5 toxicity in human lung cells in vitro. Our data revealed the benefits of tea polyphenols in attenuating the effects of PM2.5 treatment using several metrics of cellular toxicity, including indicators of oxidative stress and apoptosis. Therefore, our results identify an opportunity for developing new methods to treat PM2.5-induced toxicity, particularly with regard to designing post-exposure intervention to alleviate lung injury.
Acknowledgments
This work was supported by Hebei province postdoctoral research projects merit funding (B2015003023), Hebei province key subjects funding of medical research program (20150170) and Hebei province natural science funding (C2016106056), and DD was supported by the NIH-COBRE on Epigenomics of Development and Disease (4P20GM104360-04).
References
- Akhtar US, Rastogi N, Mcwhinney RD, Urch B, Chow CW, Evans GJ, Scott JA. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicology Reports. 2014;1:145–156. doi: 10.1016/j.toxrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K, Ebisu K, Gent JF, Lee HJ, Kouthrakis P, Leaderer BP. Prenatal exposure to fine particulate matter and birth weight. Epidemiology. 2010;21:884–891. doi: 10.1097/EDE.0b013e3181f2f405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Leaderer PB, Gent FJ, Lee HJ, Koutrakis P, Wang Y, Dominici F, Roger PD. Associations of PM2.5 constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environmental Health Perspectives. 2014;122:138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte SL, Cascella M, Schiavone V, Mehrabi-Kermani F, Cuomo A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Design, Development and Therapy. 2017;11:2737–2742. doi: 10.2147/DDDT.S142475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachon BF, Firmin S, Verdin A, Ayi-Fanou L, Billet S, Cazier F, Martin PJ, Aissi F, Courcot D, Sanni A, Shirali P. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM>2.5) collected from Cotonou, Benin. Environmental Pollution. 2014;185:340–351. doi: 10.1016/j.envpol.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Cai D, He Y. Daily lifestyles in the fog and haze weather. J Thorac Dis. 2016;8:E75–E77. doi: 10.3978/j.issn.2072-1439.2016.01.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Qin G, Shi R, Bai F, Yang G, Zhang M, Lv J. Overproduction of reactive oxygen species and activation of MAPKs are involved in apoptosis induced by PM2.5 in rat cardiac H9C2 cells. J Appl Toxicol. 2015;36:609–617. doi: 10.1002/jat.3249. [DOI] [PubMed] [Google Scholar]
- Czerska M, Mikolajewska K, Zielinski M, Gromadzinska J, Wasowicz W. Today’s oxidative stress markers. Med Pr. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Garcon G, Billet S, Gosset P, Ledoux F, Courcot D, Aboukais A, Shirali P. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225:12–24. doi: 10.1016/j.tox.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biology and Toxicology. 2013;29:143–157. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicology in Vitro. 2013;27:1762–1770. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Journal of Nutrition. 2003;133:3275–3284. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Fujiki H, Sueoka E, Rawangkan A, Suganuma M. Human cancer stem cells are a target for cancer prevention using (−)-epigallocatechin gallate. Journal of Cancer Research and Clinical Oncology. 2017;143:2401–2412. doi: 10.1007/s00432-017-2515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AM, Healy SJ, Jager R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Griess B, Tom E, Domann F, Teoh-Fitzgerald M. Extracellular superoxide dismutase and its role in cancer. Free Radical Biology and Medicine. 2017;112:464–479. doi: 10.1016/j.freeradbiomed.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Morawska L, He C, Zhang YL, Ayoko G, Cao M. Characterization of particle number concentrations and PM2.5 in a school: influence of outdoor air pollution on indoor air. Environmental Science and Pollution Research. 2010;17:1268–1278. doi: 10.1007/s11356-010-0306-2. [DOI] [PubMed] [Google Scholar]
- Hu R, Liu G, Zhang H, Xue H, Wang X, Wang R. [accessed 11 July 2017];Particle-associated polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of Hefei, China: levels, characterizations and health Risks. 2017 doi: 10.1007/s00244-017-0472-z. Http://www.who.int/gho/phe/air_pollution_pm25_concentrations/en/ [DOI] [PubMed]
- Inoue H, Akiyama S, Meada-Yamamoto M, Nesumi A, Tanaka T, Murakami A. High-dose green tea polyphenols induce nephrotoxicity in dextran sulfate sodium-induced colitis mice by down-regulation of antioxidant enzymes and heat-shock protein expressions. Cell Stress and Chaperones. 2011;16:653–662. doi: 10.1007/s12192-011-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie G, Lin Z, Zhang L, Lv H, He P, Zhao B. Free radical scavenging effect of Pu-erh tea extracts and their protective effect on oxidative damage in human fibroblast cells. J Agric Food Chem. 2006;54:8058–8064. doi: 10.1021/jf061663o. [DOI] [PubMed] [Google Scholar]
- Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HL, Nilsson L, Moller L. Subway particles are more genotoxic than street particles and induce oxidative stress in cultured human lung cells. Chem Res Toxicol. 2005;18:19–23. doi: 10.1021/tx049723c. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Lee PMY, Ng CF, Liu ZM, Ho WM, Lee MK, Wang F, Kan HD, He YH, Ng SSM, Wong SYS, Tse LA. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer Prostatic Dis. 2017 doi: 10.1038/pcan.2017.18. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Leiva GMA, Santibanez DA, Ibarra ES, Matus CP, Seguel R. A five-year study of particulate matter (PM2.5) and cerebrovascular diseases. Environmental Pollution. 2013;181:1–6. doi: 10.1016/j.envpol.2013.05.057. [DOI] [PubMed] [Google Scholar]
- Lernoux M, Schnekenburger M, Dicato M, Diederich M. Anti-cancer effects of naturally derived compounds targeting histone deacetylase 6-related pathways. Pharmacological Research. 2017 doi: 10.1016/j.phrs.2017.11.004. epub ahead of print, In press. [DOI] [PubMed] [Google Scholar]
- Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. The Journal of Nutrition. 2001;131:2248–2251. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin. 2014;46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- Liang F, Xiao Q, Wang Y, Lyapustin A, Li G, Gu D, Pan X, Liu Y. MAIAC-based long-term spatiotemporal trends of PM2.5 in Beijing, China. Science of the Total Environment. 2017 doi: 10.1016/j.scitotenv.2017.10.155. [DOI] [PubMed]
- Liu C, Hsu PC, Lee HW, Ye M, Zheng G, Liu N, Li W, Cui Y. Transparent air filter for high-efficiency PM2.5 capture. Nature Communication. 2015;6:6205. doi: 10.1038/ncomms7205. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang J, Li L, Shi W, Yuan X, Wu L. The natural occurring compounds targeting endoplasmic reticulum stress. Evid Based Complement Alternat Med. 2016;2016:7831282. doi: 10.1155/2016/7831282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jin X, Su R, Li Z. The reproductive toxicology of male SD rats after PM2.5 exposure mediated by the stimulation of endoplasmic reticulum stress. Chemosphere. 2017;189:547–555. doi: 10.1016/j.chemosphere.2017.09.082. [DOI] [PubMed] [Google Scholar]
- Lorenz M. Cellular targets for the beneficial actions of tea polyphenols. Am J Clin Nutr. 2013;98:1642–1650. doi: 10.3945/ajcn.113.058230. [DOI] [PubMed] [Google Scholar]
- Loxham M, Walsh RJM, Cooper MJ, Blume C, Swindle EJ, Dennison PW, Howarth PH, Cassee FR, Teagle DAH, Palmer MR, Davies DE. The effects on bronchial epithelial mucociliary cultures of coarse, fine, and ultrafine particulate matter from an underground railway station. Toxicol Sci. 2015;145:98–107. doi: 10.1093/toxsci/kfv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malongane F, Mcgaw LJ, Mudau FN. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J Sci Food Agric. 2017;97:4679–4689. doi: 10.1002/jsfa.8472. [DOI] [PubMed] [Google Scholar]
- Mao X, Gu C, Chen D, Yu B, He J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget. 2017;8:81649–81661. doi: 10.18632/oncotarget.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet MC, Sang S, Yang CS, Ho CT, Rosen RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem. 2004;52:2455–2461. doi: 10.1021/jf035427e. [DOI] [PubMed] [Google Scholar]
- Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Archives of Biochemistry and Biophysics. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Oh SM, Kim HR, Park YJ, Lee SY, Chung KH. Organic extracts of urban air pollution particulate matter (PM2.5)-induced genotoxicity and oxidative stress in human lung bronchial epithelial cells (BEAS-2B cells) Mutation Research. 2011;723:142–151. doi: 10.1016/j.mrgentox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Oya Y, Mondal A, Rawangkan A, Umsumarng S, Iida K, Watanabe T, Kanno M, Suzuku K, Li Z, Kagechika H, Shudo K, Fujiki H, Suganuma M. Down-regulation of histone deacetylase 4, -5 and -6 as a mechanism of synergistic enhancement of apoptosis in human lung cancer cells treated with the combination of a synthetic retinoid, AM80 and green tea catechin. Journal of Nutritional Biochemistry. 2017;42:7–16. doi: 10.1016/j.jnutbio.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Pardo M, Shafer MM, Rudich A, Schauer JJ, Rudich Y. Single exposure to near roadway particulate matter leads to confined in flammatory and defense responses: possible role of metals. Environ Sci Technol. 2015;49:8777–8785. doi: 10.1021/acs.est.5b01449. [DOI] [PubMed] [Google Scholar]
- Raza H, John A. In vitro effects of tea polyphenols on redox metabolism, oxidative stress, and apoptosis in PC12 cells. Ann N Y Acad Sci. 2008;1138:358–365. doi: 10.1196/annals.1414.037. [DOI] [PubMed] [Google Scholar]
- Renno WM, Benov L, Khan KM. Possible role of antioxidative capacity of (−)-epigallocatechin-3-gallate treatment in morphological and neurobehavioral recovery after sciatic nerve crush injury. J Neurosurg Spine. 2017;4:1–21. doi: 10.3171/2016.10.SPINE16218. [DOI] [PubMed] [Google Scholar]
- Rodriguea-Cotto RI, Ortiz-Martinez MG, Rivera-Ramirez E, Mateus VL, Amaral BS, Jimenez-Velez BD, Gioda A. Particle pollution in Rio de Janeiro, Brazil: increase and decrease of pro-inflammatory cytokines IL-6 and IL-8 in human lung cells. Environmental Pollution. 2014;194:112–120. doi: 10.1016/j.envpol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M, Naveed M, Arif M, Kakar MU, Manzoor R, Abdel-Hack ME, Alagawany M, Tiwari R, Khandia R, Munjal A, Karthik K, Dhama K, Iqbal HMN, Dadar M, Sun C. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed Pharmacother. 2017;95:1260–1275. doi: 10.1016/j.biopha.2017.09.024. [DOI] [PubMed] [Google Scholar]
- Sang S, Lambert JD, Ho C, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological Research. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Seo EJ, Wu CF, Ali Z, Wang YH, Khan SI, Walker LA, Khan IA, Efferth T. Both phenolic and non-phenolic green tea fractions inhibit migration of cancer cells. Frontiers in Pharmacology. 2016;7:398. doi: 10.3389/fphar.2016.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Qu Y, Wu Q, Han T, Gu J, Zhao J, Sun Y, Jiang Q, Gao Z, Hu M, Zhang Y, Lu K, Nordmann S, Cheng Y, Hou L, Ge H, Furuuchi M, Hata M, Liu X. Chemical characteristics of size-resolved aerosols in winter in Beijing. Journal of Environmental Sciences. 2014;26:1641–1650. doi: 10.1016/j.jes.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Sur S, Panda CK. Molecular aspects of cancer chemopreventive and therapeutic efficacies of tea and tea polyphenols. Nutrition. 2017;43–44:8–15. doi: 10.1016/j.nut.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Tahri M, Benchrif A, Bounakhla M, Benyaich F, Noach Y. Seasonal variation and risk assessment of PM2.5 and PM2.5–10 in the ambient air of Kenitra, Morocco. Environmental Science Processes & Impact. 2017 doi: 10.1039/c7em00286f. [DOI] [PubMed] [Google Scholar]
- Villar-Vidal M, Lertxundi A, Martinez Lopez De Dicastillo MD, Alvarez JI, Santa ML, Ayerdi M, Basterrechea M, Ibarluze J. Air polycyclic aromatic hydrocarbons (PAHs) associated with PM 2.5 in a North Cantabric coast urban environment. Chemosphere. 2014;99:233–238. doi: 10.1016/j.chemosphere.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Xie G, Zhao A, Zhao L, Chen T, Chen H, Qi X, Zheng X, Ni Y, Cheng Y, Lan K, Yao C, Qiu M, Jia W. Metabolic fate of tea polyphenols in humans. Journal of Proteome Research. 2012;11:3449–3457. doi: 10.1021/pr300318m. [DOI] [PubMed] [Google Scholar]
- Xu X, Qimuge A, Wang H, Xing C, Gu Y, Liu S, Xu H, Hu M, Song L. IRE1α/XBP1s branch of UPR links HIF1α activation to mediate ANGII dependent endothelial dysfunction under particulate matter (PM) 2.5 exposure. Sci Rep. 2017;7:13507. doi: 10.1038/s41598-017-13156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Villiers WJSDE, Mcclain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. The Journal of Nutrition. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- Yiannakopoulou EC. Targeting oxidative stress response by green tea polyphenols: clinical implications. Free Radical Research. 2013;47:667–671. doi: 10.3109/10715762.2013.819975. [DOI] [PubMed] [Google Scholar]
- Zuo X, Tian C, Zhao N, Ren W, Meng Y, Jin X, Zhang Y, Ding S, Ying C, Ye X. Tea polyphenols alleviate high fat and high glucose-induced endothelial hyperpermeability by attenuating ROS production via NADPH oxidase pathway. BMC Research Notes. 2014;7:120–127. doi: 10.1186/1756-0500-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]