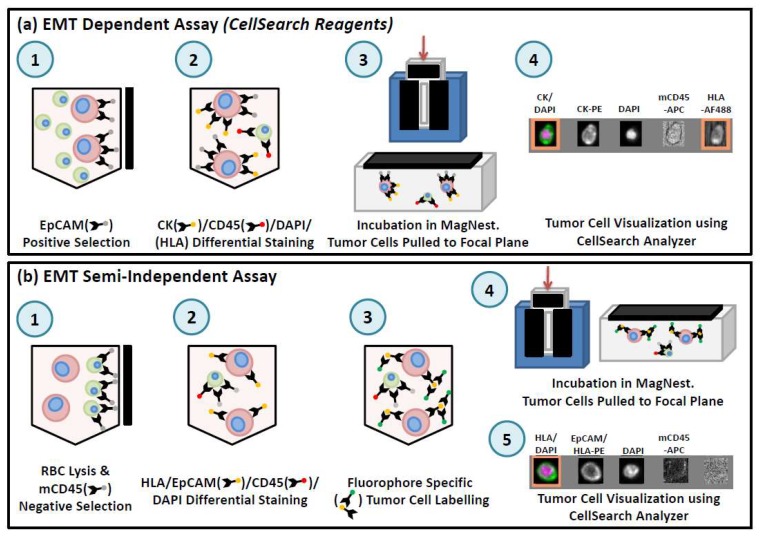
Figure 4.
Overview of workflow for preclinical CTC assays using CellSearch®. (a) For the EMT-dependent CTC assay, 50 µL of whole mouse blood is incubated with components of the CellSearch® CTC kit (anti-EpCAM ferrofluid, Capture Enhancement Reagent, Nucleic Acid Dye, Staining Reagent, Permeabilization Reagent) as well as anti-mouse CD45-APC and anti-human HLA-AlexaFluor488. Samples are manually immuno-magnetically separated and transferred to a MagNest™ for analysis using the CellSearch®. EpCAM+/CK+/DAPI+/CD45−/HLA+ cells with a round/oval morphology were classified as CTCs. (b) For the EMT semi-independent CTC assay, 50 µL of blood is lysed with NH4Cl. Samples are washed and labeled with anti-human HLA-PE, anti-human EpCAM-PE, and anti-mouse CD45-APC. Samples are washed and manually immunomagnetically enriched using the EasySep APC Positive Selection kit (StemCell Technologies, Vancouver, BC, Canada), incubated with CellSearch® Permeabilization Reagent and Nucleic Acid Dye and transferred to a MagNest™ for analysis using the CellSearch®. EpCAM/HLA+/DAPI+/CD45− cells with a round/oval morphology were classified as CTCs.