Figure 1.
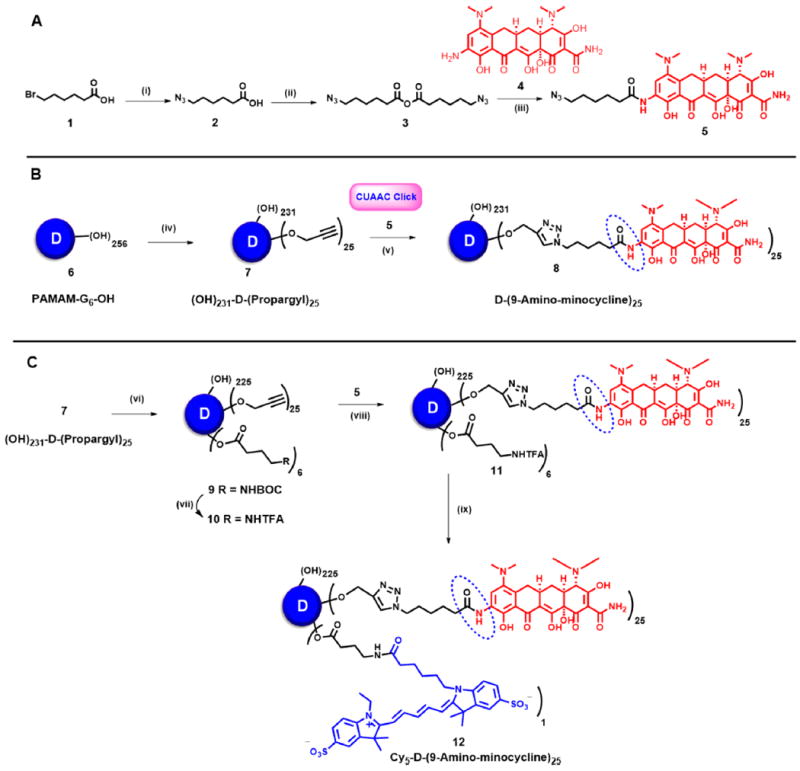
Key steps in the synthetic pathway of (A) azido-functionalized 9-amino-minocycline, 5; (B) dendrimer–9-amino-minocycline conjugate (D-mino), 8; and (C) Cy5-labeled dendrimer–9-amino-minocycline conjugate (Cy5-D-mino), 12. Reagents and conditions: (i) NaN3, DMF, 60 °C, 18 h 60%; (ii) DCC, DMF, rt, 12 h, 75%; (iii) NaHCO3, DMF, 6 h, 62%; (iv) propargyl bromide, NaH, DMF, 0 °C–rt, 12 h quantitative yield; (v) CuSO4·5H2O, Na ascorbate, DMF, THF, H2O, microwave, 40 °C, 6 h, quantitative yield; (vi) ethylene dichloride (EDC)·HCl, DMAP, Boc−GABA−OH, DMF, rt, 12 h; (vii) dichloromethane, trifluoroacetic acid; (viii) CuSO4·5H2O, Na ascorbate, DMF, THF, H2O, microwave, 40 °C; (ix) Cy5 NHS ester, DIPEA, pH 7.5, DMF, rt, 24 h.
