Abstract
Bile duct ligation (BDL) in young rats can cause impaired liver function and cognition deficits. Nitric oxide is implicated in hepatic encephalopathy and is also involved in cognition. In this study, we examined the role of brain asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, in young BDL rats with spatial deficits. Young male Sprague-Dawley rats aged 17 days were assigned to four groups: laparotomy (SHAM), laparotomy plus 5 mg melatonin delivered through a pellet (SHAMM) for 4 weeks, BDL for 4 weeks, and BDL plus 5 mg melatonin delivered through a pellet (BDLM) for 4 weeks. Their spatial memory was assessed using a Morris water-maze task. Plasma and brains were collected for biochemical and ADMA analyses. We found that the BDL group had significantly elevated levels of ADMA in the plasma, the prefrontal cortex, and the dorsal hippocampus, and worse spatial performance than that of the control groups. Melatonin administration prevented an increase in the ADMA levels in the plasma, prefrontal cortex, and dorsal hippocampus, and prevented spatial deficits in BDL rats. In addition, melatonin maintained brain-derived neurotrophic factor in the dorsal hippocampus at a level comparable with controls. We concluded that melatonin is effective in preventing spatial deficits and decreasing ADMA levels in the plasma, prefrontal cortex, and dorsal hippocampus in young BDL rats. Brain ADMA levels might play a role in BDL-induced spatial deficits.
Keywords: asymmetric dimethylarginine, bile duct ligation, melatonin, spatial memory
Introduction
Hepatic encephalopathy (HE) is a serious neuropsychiatric complication of acute liver failure or cirrhosis 1. As per the International Society for Hepatic Encephalopathy and Nitrogen Metabolism, the rodent bile duct ligation (BDL) model is an animal model of chronic liver failure associated with HE 2. BDL in rats can cause brain dysfunction, including increased oxidative/nitrosative stress 3 and cognition impairment 4,5.
Asymmetric dimethylarginine (ADMA) is a naturally occurring amino acid that can inhibit nitric oxide (NO) synthase and consequently decrease the synthesis of NO 6. NO, which is inhibited by ADMA, plays an important role in spatial memory 7. The glutamate–NO–cyclic GMP pathway is compromised in the brain of individuals with HE 8. ADMA is primarily metabolized by the liver. BDL in rats can cause increased circulating and brain ADMA concentrations 6. However, the role of ADMA in HE remains largely unknown.
Melatonin can decrease kidney and liver ADMA levels in the context of BDL 9. Melatonin (N-acetyl-5-methoxytryptamine), an effective free radical scavenger and antioxidant, is mainly secreted as a neurohormone by the pineal gland 10. Melatonin can pass the blood–brain barrier and is found to be protective after experimental traumatic brain injury 11, hypoxic brain damage 12, and HE 4.
Cognitive dysfunction is one of the most challenging complications of HE, but no specific treatment is currently available. In this study, we evaluated the effect of melatonin released through a pellet on plasma and brain ADMA levels and impaired spatial memory in young BDL rats.
Materials and methods
Animals
This experiment was conducted under the Guidelines for Animal Experiments of Chang Gung Memorial Hospital. All experiments were conducted using Sprague-Dawley rats, and day of birth was designated as postnatal day 0 (PND 0). Only male rats were used to avoid the sex effects on spatial memory and oxidative stress. At PND 17, an age equivalent to human early childhood, male Sprague-Dawley rats with an average weight about 50±5 g were operated as we reported previously 5. Attempts were made to minimize the numbers of animals used. All animals were housed in a room maintained at 24°C with 12-h light/dark cycles. All animals had free access to standard chow and tap water.
Treatment of animals and grouping
All surgical procedures were performed under ketamine (50 mg/kg) and xylazine (23 mg/kg) anesthesia with clean surgical techniques at PND 17 as described previously 4. The young BDL rats were induced by opening a midline incision and the common bile duct was ligated and divided with double ligatures of the proximal duct (BDL group, n=10). In addition, rats that received sham ligation of the bile duct were designated as the sham-control group (SHAM group, n=10). The third group included sham-operated rats that received melatonin pellet treatment (n=10, SHAMM group). Melatonin-treated rats received melatonin between PND 17 and 45 through a slow-release melatonin pellet (5 mg; Innovative Research, Sarasota, Florida, USA) implanted into the peritoneum. In addition, BDL rats that received melatonin pellets were designated as the BDLM group (n=10).
Morris water maze: spatial memory
The Morris water-maze test was performed to assess spatial learning and memory between PND 37 and 42 5,13. The water-maze was a circular pool (180 cm diameter, 50 cm high) filled with opaque water (24±1°C) to a depth of 25 cm. The invisible platform was 12 cm in diameter and made of white Plexiglas and placed 1.5 cm below the water level and equidistant from the sidewall and middle of the pool. The pool was divided into cardinal points, and the hidden platform location and spatial cues remained constant throughout experimentation. A video camera was set up above the center of the pool and connected to a video traction system (Noldus, Ethovision, The Netherlands). On the first day, PND 37, each rat was placed in the pool for 120 s without the platform to acclimatize them to the training environment. One day after acclimatization, rats were trained for six trials per day to locate and escape onto the submerged platform (PND 38–41). If a rat failed to escape within 120 s, it was placed manually on the platform. Latencies to reach the platform, the distance traveled, and the average swimming speed were recorded. Latency to reach the platform during each trial was used as a measure of acquisition. Retention of memory was evaluated on PND 42 in the absence of the platform from the pool. The percentage of time spent in the quadrant where the platform was located previously was used as a measure of retention of memory.
Measurement of plasma biochemistry parameters
After receiving BDL for 4 weeks, blood samples were collected by cardiocentesis in all four experimental groups. Rats (n=10/group) were analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), direct bilirubin, and total bilirubin according to the methods that we have published previously 4.
Tissue collection
The rats were euthanized on PND 45, and the prefrontal cortex and dorsal hippocampus were immediately collected.
Enzyme-linked immunosorbent assay
Plasma (Immunodiagnostic AG, Bensheim, Germany), prefrontal cortex, and hippocampus ADMA (Bluegene, Shanghai, China) and hippocampus brain-derived neurotrophic factor (BDNF; Bluegene) were examined using enzyme-linked immunosorbent assay kits according to the manufacturers’ instructions as we reported previously 5.
Immunohistochemistry assay
For immunohistochemical (IHC) staining, rat brain tissues were fixed in formaldehyde and dehydrated in 70% ethanol for 30 min, in 95% ethanol for 30 min, and finally in 100% ethanol for 30 min. The tissues were embedded in paraffin at 58°C, and then cut at 4–15-μm thick section using a rotary microtome. Before staining, the sections were floated in a 56°C waterbath and the sections were mounted onto slides. The slides with paraffin-embedded sections of rat brain tissue were dewaxed in xylene two times for 5 min, rehydrated in 100% ethanol for 1 min, in 90% ethanol for 1 min, and finally in 80% ethanol for 1 min. Slides with rat cortex tissues were incubated in 3% H2O2 for 1 min to remove endogenous superoxidase activity, washed with PBS, and heated with 100°C EDTA (pH 9.0) for 20 min to induce antigen retrieval. Subsequently, slides were blocked with a protein block solution and incubated with rabbit anti-ADMA antibodies for 30 min. After washing with PBS, slides were reacted with Polymer-horseradish peroxidase for 20 min, developed using the 3,3′-diaminobenzidine substrate, and counterstained with hematoxylin.
Statistical analysis
Morphologic parameters and biochemical parameters were analyzed by one-way analysis of variance (ANOVA) with the Bonferroni post-hoc test. Results from the Morris water-maze acquisition memory were evaluated using a two-way ANOVA (with group as the between-subjects factor) with repeated measures (day). The major result of the probe test (e.g. dwell time in the platform quadrant vs. all other quadrants) was compared using one-way ANOVA, followed by Bonferroni post-hoc tests. All analyses were carried out using the SPSS version 15 (IBM, Armonk, New York, USA) in a PC-compatible computer. Values were expressed as mean±SEM and significance was defined as P value less than 0.05 for all tests.
Results
Morris water maze
The water-maze tests indicated that all rats learnt how to find the platform and that there was no significant difference in swim velocity between the different treatment groups at any time (P>0.1).
Two-way ANOVA indicated that escape latencies improved over time in all four groups as shown by a significant effect of day [F(3,34)=7.984, P=0.001], indicating that learning occurred (Fig. 1a). There were significant differences among the groups for the number of trial blocks that were needed to learn to escape by swimming using visual cues [F(3,34)=7.699, P=0.001; SHAM vs. BDL, P<0.01; BDL vs. BDLM, P<0.01] (Fig. 1a). These results show that BDL caused acquisition memory deficits that melatonin could prevent.
Fig. 1.
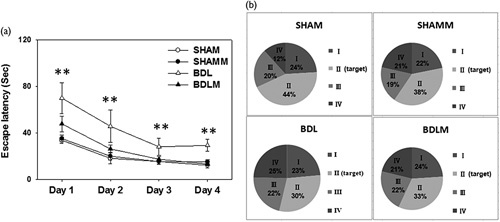
Spatial learning and memory tested by a Morris water maze. (a) Escape latencies to the platform in the Morris water maze (mean±SEM). Rats in the BDL group swam for a longer period to locate the submerged platform on all four acquisition days compared with rats from the other three groups (all P<0.001). There was no significant difference between BDLM and SHAM or SHAMM groups. (b) There were quadrant preferences in SHAM, SHAMM, and BDLM groups (all P<0.05). In contrast, there was no quadrant preference in the BDL group, indicating poor retention memory. **P<0.01 versus the SHAM group. BDL, bile duct ligation; BDLM, bile duct ligation treated with melatonin; SHAM, sham control; SHAMM, sham control treated with melatonin.
There were quadrant differences among the SHAM, SHAMM, and BDLM groups [SHAM, F(3,39)=10.445, P=0.001; SHAMM, F(3,51)=4.623, P=0.006; BDLM, F(3,23)=3.202, P=0.045] on retention in PND 41. However, there was no significant difference of retention in the target quadrant in the BDL group [F(3,23)=1.695, P=0.2]. These results showed that BDL caused retention memory deficits that were prevented by melatonin.
Plasma biochemistry parameters
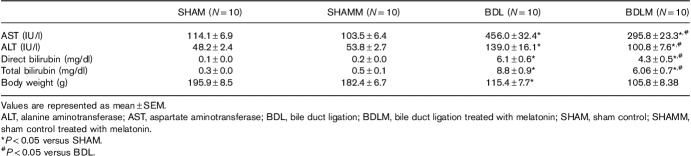
Plasma levels of direct and total bilirubin, AST, and ALT were higher in the BDL group than in the SHAM group (all P<0.05) (Table 1). However, the BDLM group had normal AST, ALT, and direct/total bilirubin levels (Table 1).
Table 1.
Clinical parameters
Asymmetric dimethylarginine enzyme-linked immunosorbent assay levels in the plasma, prefrontal cortex, and dorsal hippocampus
Plasma ADMA was significantly higher in the BDL group, but not in the BDLM group [F(3,33)=17.975, P<0.001; BDL vs. SHAM, P<0.01; BDL vs. BDLM, P<0.05] (Fig. 2). We detected higher ADMA in the prefrontal cortex of the BDL group, but not in the BDLM group [F(3,40)=15.588, P<0.01; BDL vs. SHAM, P<0.01; BDLM vs. BDL, P<0.01] (Fig. 3a). We also detected higher ADMA in the dorsal hippocampus in the BDL group, but not the BDLM group [F(3,26)=4.229, P=0.017; BDL vs. SHAM, P<0.05; BDLM vs. BDL, P<0.05] (Fig. 3b).
Fig. 2.
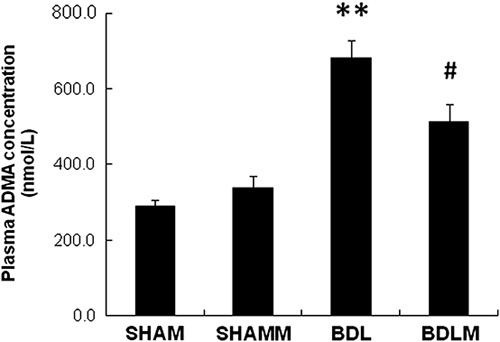
Plasma ADMA level. The BDL group had a higher plasma ADMA level than the control group. The BDLM group had lower ADMA levels compared with the BDL group, indicating the effectiveness of melatonin. **P<0.01 versus the SHAM group, #P<0.05 versus the BDLM group. ADMA, asymmetric dimethylarginine; BDL, bile duct ligation; BDLM, bile duct ligation treated with melatonin; SHAM, sham control; SHAMM, sham control treated with melatonin.
Fig. 3.
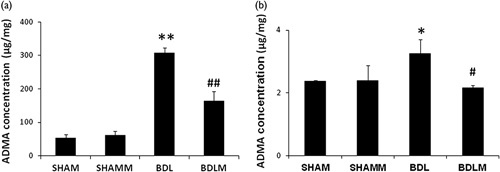
ADMA levels in the rat prefrontal cortex and dorsal hippocampus. The BDL group had higher prefrontal cortex (a) and dorsal hippocampus (b) ADMA levels than the control groups. The BDLM group had lower prefrontal cortex and dorsal hippocampus ADMA than the BDL group, indicating the effectiveness of melatonin. *P<0.05, **P<0.01 versus SHAM group; #P<0.05, ##P<0.01 versus BDL. ADMA, asymmetric dimethylarginine; BDL, bile duct ligation; BDLM, bile duct ligation treated with melatonin; SHAM, sham control; SHAMM, sham control treated with melatonin.
Brain-derived neurotrophic factor enzyme-linked immunosorbent assay levels in the dorsal hippocampus
Previous studies showed that BDNF supports synaptic plasticity and that it is critically involved in memory processes. We detected lower levels of BDNF in the dorsal hippocampus in the BDL group, but not the BDLM group [F(3,24)=8.796, P=0.021; BDL vs. SHAM, P<0.05; BDLM vs. BDL, P<0.05] (Fig. 4).
Fig. 4.
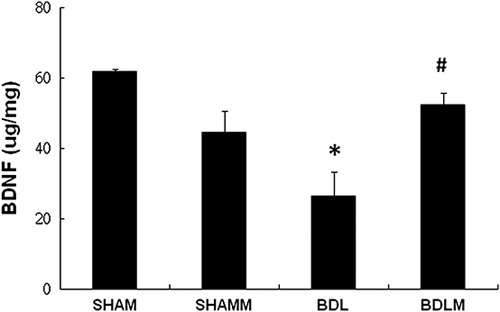
BDNF level in the rat dorsal hippocampus. The BDL group had a lower dorsal hippocampus BDNF level than the control groups. The BDLM group had higher dorsal hippocampus BDNF than the control groups, indicating the effectiveness of melatonin. *P<0.05 versus the SHAM group; #P<0.05 versus BDL. BDL, bile duct ligation; BDLM, bile duct ligation treated with melatonin; BDNF, brain-derived neurotrophic factor; SHAM, sham control; SHAMM, sham control treated with melatonin.
Immunohistochemical staining of asymmetric dimethylarginine in the brain
IHC staining showed that the ADMA levels were higher in the BDL group than in the SHAM group. Moreover, melatonin treatment restored brain ADMA in the BDLM group [Fig. 5, F(3,12)=3.451, P=0.01; BDL ∼1.4-fold of SHAM; BDL vs. SHAM, P<0.01; BDLM vs. BDL, P<0.05].
Fig. 5.
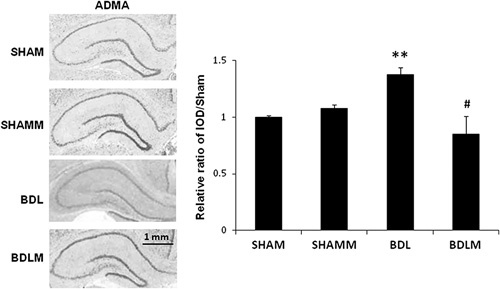
Immunohistochemical staining of ADMA in rat brain. The ADMA level was decreased in the BDL rats. However, melatonin treatment prevented the decrease in ADMA levels (the BDLM group). The IOD in the positive areas of the rat cortex was measured using Image-Pro Plus 6.0 software. The IOD/area values of ADMA expression are shown in the histogram. **P<0.001 versus the SHAM group; #P<0.05 versus the BDL group. N=3 in each group. Scale bar=100 µm. ADMA, asymmetric dimethylarginine; BDL, bile duct ligation; BDLM, bile duct ligation treated with melatonin; IOD, integrated optical density; SHAM, sham control; SHAMM, sham control treated with melatonin.
Discussion
The main findings of this study were as follows: (i) melatonin effectively prevented increases of ADMA in the plasma, prefrontal cortex, and dorsal hippocampus in young BDL rats; (ii) melatonin effectively upregulated the levels of BDNF in the dorsal hippocampus in young BDL rats; and (iii) melatonin effectively prevented a decline in spatial acquisition and retention memory deficits in young BDL rats.
A previous study showed an association between ADMA levels in the cerebrospinal fluid and cerebral vasospasm in a primate model of subarachnoid hemorrhage 14. Conceivably, ADMA may contribute toward cerebral vasospasm and affect cognition 15. Interestingly, epidemiological studies support a potential link between ADMA and cerebrovascular disease and cognitive impairment 16. Therefore, agents that can lower ADMA are targets of current research.
Balasubramaniyan et al. 17 reported that brain ADMA levels were significantly higher in adult rats 4 weeks after BDL and their ADMA levels were reduced after treatment with ornithine phenylacetate. Similarly, Bajaj et al. 18 reported that patients with liver cirrhosis showed cognition dysfunction and higher serum ADMA levels. We have previously shown that rats with BDL for 2 weeks had increased plasma ADMA levels as well as cognitive deficits 19. Here, we replicate our previous findings, showing increased ADMA levels in the prefrontal cortex in rats 4 weeks after BDL 5. The prefrontal cortex plays a role in memory, attentiveness, and perceptual awareness. The dorsal hippocampus and ventral hippocampus have distinct neuroanatomical profiles, whereas the dorsal hippocampus is primarily involved in cognition 20. Spatial memory needs a wider network of inter-connected brain regions including the prefrontal cortex and the hippocampus. Rats with lesions of the prefrontal cortex or the hippocampus show spatial deficits 21. In our previous study, the entire hippocampus did not show a significant difference in ADMA levels between controls and 4-week BDL rats 5. Here, we found increased ADMA concentration in the dorsal hippocampus in 4-week BDL rats, that is, the BDL group, and melatonin infusion therapy prevented the increase in ADMA. In addition, IHC staining showed a protective effect of melatonin against increased hippocampal ADMA expression. To the best of our knowledge, this is the first report on the protective effect of melatonin against increases of ADMA levels in the dorsal hippocampus in a brain disorder.
BDNF supports synaptic plasticity and is critically involved in memory processing. Previous reports have shown decreased hippocampal BDNF mRNA in adult BDL rats 22 and decreased prefrontal cortex BDNF mRNA in young BDL rats 13. Here, we showed a decrease in BDNF expression in BDL rats’ dorsal hippocampus that was prevented by melatonin, suggesting a role of BDNF in cognition deficits in BDL rats.
Increased oxidative stress can interfere with BDNF expression. Oxidative stress may decrease the DNA-binding activities of activator protein-1 and cAMP response element binding protein, and thus decrease BDNF gene expression 23.
ADMA is an oxidant and could therefore possibly interfere with BDNF synthesis. Interestingly, a 4-week ADMA infusion in healthy rats was shown to induce a marked reduction in serum BDNF levels 24. This study thus provides supportive evidence that ADMA may affect cognition through downregulation of BDNF.
We have previously shown that melatonin prevented spatial deficits in young rats with BDL for 2 weeks 4. We also found alterations in NADPH oxidase expression and blood–brain barrier in the prefrontal cortex and hippocampus in young BDL rats 25. Here, we showed that melatonin released through a pellet could prevent spatial deficits in BDL rats by downregulation of circulating and brain ADMA and upregulation of dorsal hippocampus BDNF. In addition, melatonin prevented increases in plasma AST, ALT, and direct/total bilirubin levels in these rats.
Conclusion
We found that BDL in young rats cause spatial deficits and increased ADMA in the plasma, prefrontal cortex, and dorsal hippocampus. Melatonin released slowly through a pellet prevented changes in circulating and brain ADMA and prevented the development of any spatial deficit. These results could improve our understanding of the molecular mechanisms associated with HE and open new avenues for its treatment.
Acknowledgements
This work was supported by grant CMRPG8F0182, CMRPG8F0111, CMRPG8G0441, and CMRPG8G0631 from Chang Gung Memorial Hospital, Kaohsiung, Taiwan, to Dr Li-Tung Huang.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Souto PA, Marcotegui AR, Orbea L, Skerl J, Perazzo JC. Hepatic encephalopathy: Ever closer to its big bang. World J Gastroenterol 2016; 22:9251–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 2009; 29:783–788. [DOI] [PubMed] [Google Scholar]
- 3.Gorg B, Schliess F, Haussinger D. Osmotic and oxidative/nitrosative stress in ammonia toxicity and hepatic encephalopathy. Arch Biochem Biophys 2013; 536:158–163. [DOI] [PubMed] [Google Scholar]
- 4.Huang LT, Tiao MM, Tain YL, Chen CC, Hsieh CS. Melatonin ameliorates bile duct ligation-induced systemic oxidative stress and spatial memory deficits in developing rats. Pediatr Res 2009; 65:176–180. [DOI] [PubMed] [Google Scholar]
- 5.Sheen JM, Chen YC, Hsu MH, Tain YL, Yu HR, Huang LT. Combined intraperitoneal and intrathecal etanercept reduce increased brain tumor necrosis factor-alpha and asymmetric dimethylarginine levels and rescues spatial deficits in young rats after bile duct ligation. Front Cell Neurosci 2016; 10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheen JM, Chen YC, Tain YL, Huang LT. Increased circulatory asymmetric dimethylarginine and multiple organ failure: bile duct ligation in rat as a model. Int J Mol Sci 2014; 15:3989–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab 2005; 25:663–672. [DOI] [PubMed] [Google Scholar]
- 8.Corbalán R, Montoliu C, Miñana MD, Del Olmo JA, Serra MA, Aparisi L, et al. Altered modulation of soluble guanylatecyclase by nitric oxide in patients with liver disease. Metab Brain Dis 2002; 17:295–301. [DOI] [PubMed] [Google Scholar]
- 9.Tain YL, Hsieh CS, Chen CC, Sheen JM, Lee CT, Huang LT. Melatonin prevents increased asymmetric dimethylarginine in young rats with bile duct ligation. J Pineal Res 2010; 48:212–221. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004; 36:1–9. [DOI] [PubMed] [Google Scholar]
- 11.Kerman M, Cirak B, Ozguner MF, Dagtekin A, Sutcu R, Altuntas I, et al. Does melatonin protect or treat brain damage from traumatic oxidative stress? Exp Brain Res 2005; 163:406–410. [DOI] [PubMed] [Google Scholar]
- 12.Tutunculer F, Eskiocak S, Basaran UN, Ekuklu G, Ayvaz S, Vatansever U. The protective role of melatonin in experimental hypoxic brain damage. Pediatr Int 2005; 47:434–439. [DOI] [PubMed] [Google Scholar]
- 13.Li SW, Chen YC, Sheen JM, Hsu MH, Tain YL, Chang KA, et al. Minocycline restores cognitive-relative altered proteins in young bile duct-ligated rat prefrontal cortex. Life Sci 2017; 180:75–82. [DOI] [PubMed] [Google Scholar]
- 14.Jung CS, Iuliano BA, Harvey-White J, Espey MG, Oldfield EH, Pluta RM. Association between cerebrospinal fluid levels of asymmetric dimethyl-l-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg 2004; 101:836–842. [DOI] [PubMed] [Google Scholar]
- 15.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther 2005; 105:23–56. [DOI] [PubMed] [Google Scholar]
- 16.Notsu Y, Nabika T, Bokura H, Suyama Y, Kobayashi S, Yamaguchi S, et al. Evaluation of asymmetric dimethylarginine and homocysteine in microangiopathy related cerebral damage. Am J Hypertens 2009; 22:257–262. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramaniyan V, Wright G, Sharma V, Davies NA, Sharifi Y, Habtesion A, et al. Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am J Physiol Gastrointest Liver Physiol 2012; 302:G145–G152. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS, Ahluwalia V, Wade JB, Sanyal AJ, White MB, Noble NA, et al. Asymmetric dimethylarginine is strongly associated with cognitive dysfunction and brain MR spectroscopic abnormalities in cirrhosis. J Hepatol 2013; 58:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang LT, Chen CC, Sheen JM, Chen YJ, Hsieh CS, Tain YL. The interaction between high ammonia diet and bile duct ligation in developing rats: assessment by spatial memory and asymmetric dimethylarginine. Int J Dev Neurosci 2010; 28:169–174. [DOI] [PubMed] [Google Scholar]
- 20.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vann SD, Albasser MM. Hippocampus and neocortex: recognition and spatial memory. Curr Opin Neurobiol 2011; 21:440–445. [DOI] [PubMed] [Google Scholar]
- 22.Dastgheib M, Dehpour AR, Heidari M, Moezi L. The effects of intra-dorsal hippocampus infusion of pregnenolone sulfate on memory function and hippocampal BDNF mRNA expression of biliary cirrhosis-induced memory impairment in rats. Neuroscience 2015; 306:1–9. [DOI] [PubMed] [Google Scholar]
- 23.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 2004; 19:1699–1707. [DOI] [PubMed] [Google Scholar]
- 24.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martenslobenhoffer J, et al. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 2006; 37:2024–2029. [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, Sheen JM, Tain YL, Chen CC, Tiao MM, Huang YH, et al. Alterations in NADPH oxidase expression and blood–brain barrier in bile duct ligation-treated young rats: effects of melatonin. Neurochem Int 2012; 60:751–758. [DOI] [PubMed] [Google Scholar]