Abstract
Background
Nipple-sparing mastectomy (NSM) offers several advantages for women seeking post-mastectomy breast reconstruction, but compromised skin and nipple perfusion may lead to skin and nipple necrosis. It is unclear if the incisional approach contributes to these complications; therefore, the purpose of this study was to compare the impact of incision type on outcomes in patients undergoing NSM.
Methods
This is a prospective cohort study of patients undergoing NSM with prosthetic breast reconstruction through an inframammary fold (IMF) versus a lateral radial (LR) incision. Skin and nipple perfusion as represented by fluorescent intensity, mammometric parameters, patient-reported outcomes, and clinical outcomes were analyzed and compared for the two cohorts and multivariable logistic regression models were performed to evaluate effects of covariates on outcomes.
Results
79 patients were studied: 55 in the IMF cohort and 24 in the LR cohort. The IMF group had significantly less fluorescent intensity to the inferior (21.9% vs 36.9%, p=0.001) and lateral portions of breast skin (23.1% vs 40.7%, p=0.003) after reconstruction. Decreased fluorescent intensity was associated with smoking, decreased mean arterial pressure, and greater specimen weight. Post-reconstruction breast volumes were increased over preoperative volumes in the IMF group (38.3%) versus the LR (31.2%) group; however patients with a LR incision had a greater increase in satisfaction with their breasts and psychosocial well-being.
Conclusions
There are significant differences in patient-reported outcomes and final breast volumes based on the incisional approach to NSM. These data can be used to guide providers and counsel patients considering NSM with prosthetic reconstruction.
INTRODUCTION
In the United States, rates of mastectomy and contralateral prophylactic mastectomy have steadily increased compared to breast conservation surgery,(1-3) concurrent with an increase in rates of nipple sparing mastectomy (NSM) procedures.(4-10) Preservation of the nipple-areolar complex with NSM offers the potential for improved aesthetic outcomes and health-related quality of life in women who opt for postmastectomy breast reconstruction. In fact, over the last several years, multiple studies demonstrate that NSM with immediate prosthetic (two-stage tissue expander-implant or direct-to-implant) breast reconstruction improves women’s psychosocial and sexual well-being as well as their satisfaction with appearance and body image. (11-13)
While initial clinical and patient-reported outcomes (PRO) have been encouraging, optimizing outcomes in NSM with breast reconstruction remains a challenge. Complications following NSM, including nipple loss, have significant adverse effects on patients and are primarily attributable to poor skin and nipple perfusion. Prior to mastectomy, perfusion to the breast is primarily derived from the internal thoracic artery with contributions from the lateral thoracic, thoracoacromial and anterior intercostal vessels, (14-18) although extension to the nipple and subareolar dermal plexus is less well defined.(18, 19) NSM creates random pattern flaps whose perfusion pattern is not well characterized. Still, analysis of pre- and postmastectomy angiography suggests that preoperative perfusion patterns may impact post-NSM nipple perfusion,(20) and perfusion of the nipple-areola complex may be improved by preservation of the internal thoracic artery.(21)The lateral radial (LR) and inframammary fold (IMF) incisions are most commonly used for NSM with lower rates of necrosis compared with other surgical incisions.(22-27) An IMF incision has the potential to spare blood supply from the internal thoracic arteriey, but may disrupt the hypervascular zone anterior to the inferior border of the pectoralis major.(14) In a retrospective analysis of 285 women treated with NSM, the IMF incision was associated with fewer complications.(28)
To date, few studies have evaluated the impact of incision type on complications and outcomes in prosthetic breast reconstruction after NSM using validated and objective measures. Furthermore, there is minimal data on how incision type affects PRO in this population. To improve the ability of clinicians to provide patient-centered care while optimizing postoperative outcomes and patient health-related quality of life, we sought to compare both clinical and PRO for patients undergoing NSM with prosthetic breast reconstruction. We hypothesize the IMF incisional approach, while a more technically challenging procedure,(29) will offer the most favorable combination of breast perfusion and aesthetic outcomes, thus minimizing complications while maximizing patient satisfaction.
METHODS
Study Population and Design
This study is a prospective clinical trial of women undergoing NSM with prosthetic (tissue-expander or implant-based) breast reconstruction at a National Cancer Institute designated Comprehensive Cancer Center from April 2013 to April 2016. This trial was approved by the Institutional Review Board (201302004) and registered with Clinical Trials.gov (NCT01969448). Women were eligibile for inclusion if they were scheduled to undergo NSM for breast cancer or cancer prevention, had a Karnofsky Performance Scale Score (KPS) of >80, and were > 18 years old.(30) Study design and exclusion criteria are summarized in Figure 1. Initially, this study had a randomized-controlled trial design with assignment to an IMF or a LR incision; however, during trial enrollment patients frequently withdrew from randomization due to strong patient or surgical oncologist preference and/or pre-existing scars precluding randomization (See Figure, Supplemental Digital Content 1, which shows of 59 patients in the non-randomized cohort, 38 patients had an IMF incision. This was based on patient preference in 27 cases (71% of non-randomized IMF incision cases) and oncologic surgeon bias based on pre-existing scars or oncologic reasons in 11 cases (29%). The LR incision was deliberately selected by patients in 5 cases (56% if non-randomized LR incision cases), and by the oncologic surgeon in the remainder. In 32 cases where incision selection was patient-driven and randomization was refused, patients selected the IMF incision over the LR incision in 84% of cases, INSERT HYPER LINK). Therefore, data are analyzed as a prospective trial without randomization. Metrics including applanation tonometry, mammometrics, patient reported and clinical outcomes are summarized in Table 1.
Figure 1.
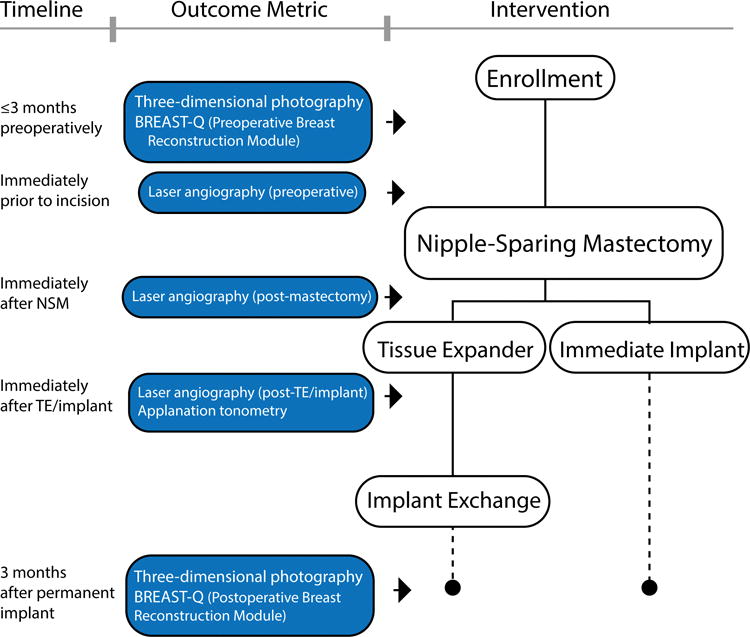
Schematic of study design. Patients were excluded if they had no reconstruction, autologous flap reconstruction, prior radiation therapy, a body mass index less than 18 or greater than 35, breast size <100g or >800g by mammometric analysis (Vectra XT, Canfield Scientific Inc, Parsippany, NJ), or an allergy to indocyanine green (ICG). In bilateral cases, a “study breast” was randomly assigned for perfusion analysis. Patients who were lost to follow-up were excluded from analysis. Three patients were excluded due to poor quality perfusion imaging. Seven patients did not have both pre- and post-operative three-dimensional imaging and were excluded from mammometric analysis. Finally, seven patients did not have preoperative Q-score and 12 did not have post-operative Q-scores available.
Table 1.
Summary of mammometrics, applanation tonmetry, and patient reported outcomes.
| Outcome Metric | Methodology |
|---|---|
| Applanation Tonometry | Quantifies intramammary compliance of breasts preoperatively and after initial prosthetic reconstruction. Intramammary compliance is the force with which breast implants “push back” against the skin envelope, thereby extrinsically compressing the subcutaneous blood supply.(54, 55) Performed preooperativey and immediately after placement of implant or tissue expander with initial fill volume. Applanation tonotmetry correlated with implant size and complications. Mean arterial pressure (MAP) was used as a partial correlation coefficient and was measured at the beginning of the reconstruction. |
| Mammometrics | Preoperative photographs were taken with the Vectra camera system and breast surface area and volume calculated using the Vectra Analysis Module (Canfield Scientific Inc, Parsippany, NJ). Landmarks, including the clavicle, sternal notch (SN), medial and lateral breast folds, IMF, nipple, and areola, were manually placed on the images. The total volume to the interpolating surface (chest wall), nipple-IMF, SN-nipple, internipple distances, and base width were calculated and recorded for each patient. Images were taken throughout the post-operative period and final imaging after revision surgeries was used for post-operative measurements. |
| Patient Reported Outcomes | Pre- and postoperative Breast-Q reconstructive modules used to measure changes in health-related quality of life.(56) Where pertinent, change in Breast-Q from the pre- to postoperative state is reported.(57-59) |
| Clinical Outcomes | Wound dehiscence, necrosis, infection, seroma, or implant exposure were recorded for 12 weeks postoperatively. Partial nipple necrosis was defined as partial-thickness compromise not requiring operative intervention. Nipple and mastectomy flap loss were characterized by full-thickness loss requiring debridement or excision. Device malposition was documented in any case where there was a pocket modification such as a capsulotomy or capsulorrhaphy at the time of implant exchange or revision of a permanent implant during the study period. |
Operative Procedure
Mastectomies were performed through an IMF or LR incision (Figure 2) by two surgical breast oncologists (JAM and AEC) and reconstructions by two plastic surgeons (TMM and MMT). Post-pectoral tissue expanders or silicone breast implants and acellular dermal matrix (ADM) were used for postmastectomy reconstruction.
Figure 2.

Incision location on three dimensional breast images and Spy laser-assisted angiography. A. Inframammary incision (IMF) in blue. B. Lateral radial incision (LR) in red. C. Greyscale static image of perfusion following inframammary approach. “IMF” denotes inframammary fold incision. “N” denotes nipple. D. Color coded version of image demonstrates greater perfusion medially from internal mammary artery perforators labeled “IMA”. Limited blood supply in inferior breast approaching nipple in similar distribution to surgical retractor placement. “RI” denotes retractor injury.
Perfusion Imaging
Intraoperative laser angiography using the Spy Elite device (Novadaq Technologies Inc, Bonita Springs, Florida) was performed with standardized focal length, orientation, and lighting. Fluorescent intensity (FI), measured using an 8-bit greyscale (pixel intensity 0-255) with baseline intensity subtracted, was used as a marker of perfusion. To standardize image acquisition to baseline values and environmental factors, we report percentage change from preoperative imaging for each region of interest. Total ingress was represented by cumulative FI (area under the curve), time to peak FI was measured in seconds, and the rate of perfusion was measured as the change in FI over time. Mean arterial pressure was recorded immediately prior to reconstruction. Spy imaging was used as a study tool to measure perfusion, but we did not resect tissue based on its findings.
FI was used to represent measured perfusion i) before NSM (pre-NSM) ii) following NSM but before reconstruction (post-NSM), and iii) after reconstruction with placement of the implant or tissue expander inflated to its initial fill volume (post-reconstruction). As only a single breast could be analyzed at once, in bilateral reconstructions the imaged breast was randomly assigned. Angiograms were separated by at least 20 minutes to optimize egress of the ICG. Fluorescence was captured for 90 seconds starting immediately after administration of 12.5 mg ICG intravenously. SpyQ Version 1.2 software (Novadaq, Mississauga, ON) was used for analysis.
Statistical Analysis
A two-sample two-tailed T-test, Mann-Whitney U, or Fisher Exact test was performed to ensure homogeneity in demographics, comorbidities, and operative factors between groups. FI was analyzed for the whole breast and regions of interest at the three described time points using a two-tailed ANOVA test with Bonferroni correction. Complication rates, mammometric data, and Q-Scores were analyzed using a two-tailed t-test and Fisher Exact test. Backwards stepwise elimination was used to generate a linear regression model to predict perfusion based on patient factors. Covariates included smoking status, age, specimen weight, laterality, mean arterial pressure (MAP) and incision type. Logistic regression was used to assess the impact of covariates on complications, and again backwards stepwise elimination was used to remove non-significant variables. Perfusion was treated as an outcome following NSM and as a covariate thereafter to predict clinical complications. Any missing or incomplete data were excluded from analysis. Pearson’s correlation coefficient was used to demonstrate non-causal relationships between variables. A p<0.05 indicated significance. Statistical analyses were performed in SAS Version 9.4 of the SAS System (Cary, NC).
RESULTS
Patient demographics and operative characteristics
Seventy-nine patients comprised the study population, with 55 in the IMF and 24 in the LR incision group. Their descriptive statistics are summarized in Table 2. There were no differences between groups with respect to demographic, operative variables, or perfusion pattern.
Table 2.
Summary statistics, operative variables, and perfusion patterns.
| Summary Statistics | ||||
|---|---|---|---|---|
| All patients (n=79) | IMF (n=55) | LR (n=24) | p | |
| Age, years | 44.2 ± 10.7 | 43.2 ± 10.8 | 46.6 ± 10.5 | 0.2 |
| BMI, kg/m2 | 23.6 ± 3.2 | 2]3.3 ± 3.1 | 24.2 ± 3.7 | 0.3 |
| KPS* | 96.7 ± 4.3 | 96.5 ± 4.8 | 97.1 ± 4.6 | 0.7 |
| Current smoker | 7 (8.9%) | 6 (10.91%) | 1 (4.2%) | 0.7 |
| Caucasian | 77 (97.5%) | 54 (98.2%) | 23 (95.8%) | 1 |
| Prior radiation** | 0 | 0 | 0 | 1 |
| Operative Variables | ||||
| SBP (mmHg) | 102.9 ± 8.4 | 102.7 ± 8.6 | 103.3 ± 7.9 | 0.8 |
| MAP (mmHg) | 73.8 ± 6.9 | 73.8 ± 6.9 | 76.2 ± 6.5 | 0.1 |
| Specimen weight, grams | 371± 157 | 350 ± 156 | 418 ± 151 | 0.1 |
| Breast surface area, cm2 | 209.2 ± 61.4 | 201.1 ± 65.5 | 218.1 ± 195.5 | 0.4 |
| Study breast, R | 53 (67.1%) | 38 (69.1%) | 15 (62.5%) | 0.6 |
| Incision length, cm | 10.0 ± 2.5 | 11.3 ± 1.9 | 7.3 ± 1.1 | <0.001 |
| Prophylactic | 46 (58.2%) | 31 (56.4%) | 16 (66.7%) | 0.6 |
| Bilateral | 71 (89.9%) | 49 (89.1%) | 22 (91.7%) | 0.8 |
| Operative time, min | 161.9 ± 39.1 | 155.3 ± 41.2 | 177.0 ± 29.0 | 0.01 |
| Direct to implant | 48 (60.8%) | 36 (65.5%) | 12 (50.0%) | 0.2 |
| Shaped implant | 45 (94.8%) | 33 (91.7%) | 12 (100%) | 0.6 |
| Initial volume, mL*** | 361.6±169.4 | 369.5±171.3 | 343.8±167.1 | 0.5 |
| Final implant volume,mL | 489.9±129.1 | 511.1±124.1 | 441.5±129.6 | 0.03 |
| Implant base width | 13.5±1.15 | 13.6±1.3 | 13.2±1.2 | 0.1 |
| Implant height | 13.5±1.42 | 13.7±1.3 | 13.0±1.5 | 0.03 |
| Implant projection | 5.7±0.61 | 5.8±0.6 | 5.5±0.2 | 0.04 |
| Preoperative tonometry N/m2 | 579.2±283.1 | 581.4±278.7 | 573.9±294.3 | 0.9 |
| Postoperative tonometry N/m2 | 1542.7±1140.6 | 1718.7±1329.8 | 1164.3±339.7 | 0.01 |
| Perfusion Pattern | ||||
| V1 | 27 (34.2%) | 18 (32.7%) | 9 (37.5%) | 0.2 |
| V2 | 25 (31.7%) | 18 (32.7%) | 7 (29.2%) | 0.1 |
| V3 | 27 (34.2%) | 19 (34.6%) | 8 (33.3%) | 1 |
Karnofsky Performance Score (KPS)
Prior radiation refers specifically to the study breast.
Initial volume refers to volume of the implanted device in direct-to-implant cases, and the initial fill volume for tissue expander cases.
Breast Perfusion - Pattern
NAC and skin envelope perfusion was characterized by perfusion pattern characterized by the Spy device: V1 by perforators traversing the breast parenchyma; V2 by inflow traversing the superficial subcutaneous tissue; and V3 by a combination of V1 and V2 (20). Breasts were evenly distributed between V1 (n=27), V2 (n=25) and V3 (n=27) patterns (Table 2). Preoperative perfusion pattern did not significantly impact remaining post-reconstruction perfusion pattern to the breast, nipple, inferior, or lateral regions or resulting complication rates (Table 3).
Table 3.
Fluorescent Intensity and complication rates by perfusion pattern.*
| Fluorescent Intensity by Perfusion Pattern | |||||
|---|---|---|---|---|---|
| All patients | V1 (n=27) | V2 (n=25) | V3 (n=27) | p | |
| Post-NSM, breast overall | 62.9 ± 26.1 % | 64.0 ± 23.6% | 62.2 ± 22.8% | 62.5 ± 31.7% | 0.96 |
| Post-recon, breast overall | 31.9 ± 14.4% | 32.5 ± 14.0% | 31.3 ± 9.0% | 31.9 ± 18.5% | 0.96 |
| Post-NSM, nipple | 33.0 ± 26.7% | 34.2 ± 23.3% | 36.3 ± 24.1% | 28.9 ± 24% | 0.6 |
| Post recon, nipple | 25.7 ± 23.07% | 26.4 ± 20.2% | 26.1 ± 22.5% | 24.6 ± 26.8% | 0.95 |
| Post-NSM, inferior | 59.8 ± 32.3% | 58.3 ± 30.4% | 65.5 ± 32.9% | 56.1 ± 34.1% | 0.6 |
| Post-recon, inferior | 26.5 ± 19.4% | 26.8 ± 15.6% | 28.6 ± 22.4% | 24.2 ± 20.3% | 0.7 |
| Post-NSM, lateral | 74.4 ± 49.0% | 75.9 ± 47.5% | 85.9 ± 64.8% | 63.1 ± 29.0% | 0.2 |
| Post-recon, lateral | 28.4 ± 20.6% | 27.0 ± 20.4% | 32.4 ± 19.3% | 26.2 ± 22.2% | 0.5 |
| Post-NSM, medial | 63.3 ± 31.3% | 65.6 ± 29.4% | 60.7 ± 28.6% | 63.5 ± 36.2% | 0.9 |
| Post-recon, medial | 29.2 ± 18.4% | 31.4 ± 16.7% | 25.6 ± 16.6% | 28.6 ± 21.6% | 0.7 |
| Complications by Perfusion Pattern | |||||
| Any Necrosis | 21 (26.5%) | 8 (29.6%) | 4 (16%) | 9 (33.3%) | 0.3 |
| Nipple tip necrosis | 16 (20.2%) | 6 (22.2%) | 2 (8%) | 8 (29.6%) | 0.2 |
| Nipple loss | 1 (1.3%) | 1 (3.7%) | 0 | 0 | 1 |
| Flap necrosis requiring excision | 8 (10.1%) | 4 (14.8%) | 3 (12%) | 1 (3.7%) | 0.4 |
| Infection | 8 (10.1%) | 3 (11.1%) | 3 (12%) | 2 (7.4%) | 0.9 |
| Hematoma | 3 (3.8%) | 2 (7.4%) | 1 (4%) | 0 | 0.5 |
| Seroma | 5 (6.3%) | 2 (7.4%) | 2 (8%) | 1 (3.7%) | 0.9 |
| Device malposition | 6 (7.6%) | 2 (7.4%) | 3 (12%) | 1 (3.7%) | 0.5 |
| Implant exposure | 0 | 0 | 0 | 0 | 1 |
| Wound dehiscence | 2 (2.5%) | 1 (3.7%) | 0 | 1 (3.7%) | 1 |
| Total OR trips | 1.6 ± 0.7 | 1.7 ± 0.7 | 1.7 ± 0.7 | 1.3 ± 0.7 | 0.1 |
| Explant | 4 (5.1%) | 1 (3.7%) | 3 (12%) | 0 | 0.1 |
Values presented as a percentage of the original preoperative perfusion, minus background fluorescence, based on 0-255 unit, 8-bit greyscale.
Breast Perfusion – Surgery, Incision, Region of Interest
Preoperative maximal perfusion, based on a FI range of 0-255, was 149.2 units for the whole breast, 223.4 for the nipple, 132.1 for the inferior, 188.9 for the medial, and 92.9 for the lateral skin (Figure 3). NSM reduced mastectomy skin flap FI to 62.9 ± 26.1% of its original value (Table 4) and was not significantly impacted by incision. Nipple FI was most affected by NSM, dropping to 33.0 ± 26.7% of preoperative values. Reconstruction reduced whole breast FI down to 31.9 ± 14.4% of its original value, but had less impact on nipple FI, dropping <8% (25.7 ± 23.1%). FI to the inferior and lateral regions of the breast was more dramatically impacted by reconstruction (Table 4). There was significantly less FI to the inferior region after mastectomy, and to the lateral and inferior regions in the IMF group after reconstruction (Table 4).
Figure 3.
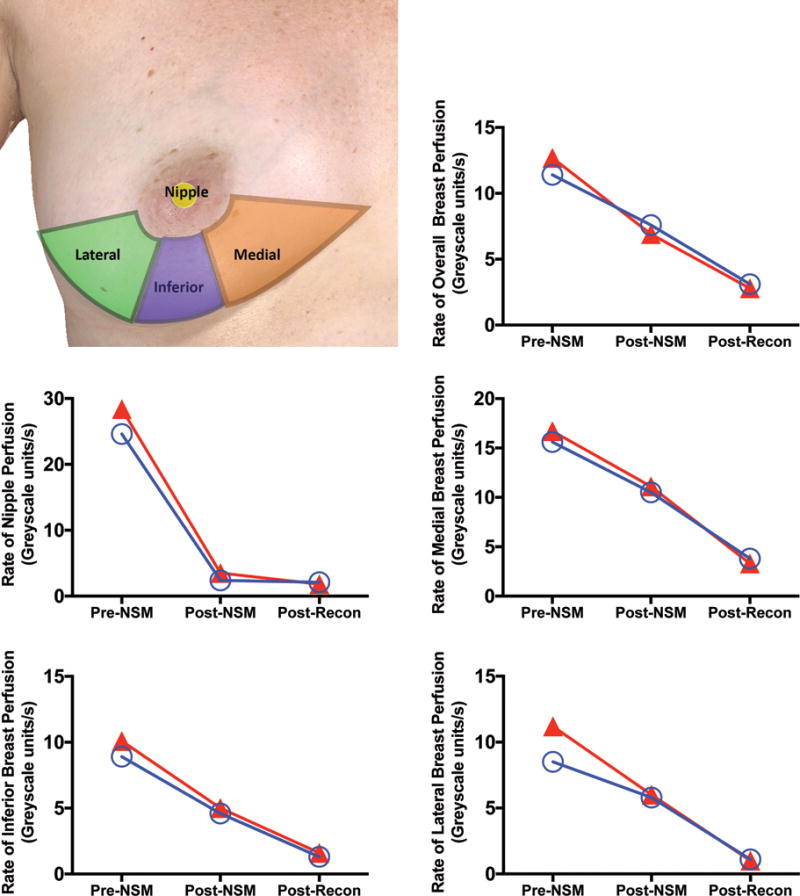
Rate of nipple perfusion by breast region prior to surgery, after nipple-sparing mastectomy (NSM) and following mastectomy. A. Breast regions of interest. The inferior region of intrerest was bordered by the the central third of the caudal circumference of the areola and extended to the central third of the inframammary fold. The medial and lateral regions of interest were bordered by the inferior region of interest, the medial/lateral/inframammary fold, and a line that bisected the breast transversely with the patient lying supine. Overall breast perfusion represented the overall area captured by the camera. Mean breast perfusion, presented as 8-bit greyscale units/second, for the inframammary fold (IMF) and lateral radial (LR) groups pre-NSM, post-NSM, and post-reconstruction are presented. Rates of perfusion for the regions of interest include B. overall, C. nipple, D. medial, E. inferior, and F. lateral breast.
Table 4.
Remaining fluorescent intensity and complications after nipple-sparing mastectomy and prosthetic breast reconstruction.*
| Fluorescent Intensity by Incision Type | ||||
|---|---|---|---|---|
| All patients (n=79) | IMF (n=55) | LR (n=24) | P | |
| Post-NSM, breast overall | 62.9 ± 26.1 % | 63.9 ± 26.6% | 60.8 ± 25.4% | 0.6 |
| Post-recon, breast overall | 31.9 ± 14.4% | 30.6 ± 13.0% | 35.1 ± 17. % | 0.2 |
| Post-NSM, nipple | 33.0 ± 26.7% | 29.9 ± 24.2% | 40.0 ± 31.1% | 0.1 |
| Post recon, nipple | 25.7 ± 23.07% | 23.2 ± 22.0% | 31.3 ± 24.9% | 0.2 |
| Post-NSM, inferior | 59.8 ± 32.3% | 54.6 ± 32.2% | 72.0 ± 30.0% | 0.02 |
| Post-recon, inferior | 26.5 ± 19.4% | 21.9 ± 17.6% | 36.9 ± 22.6% | 0.001 |
| Post-NSM, lateral | 74.4 ± 49.0% | 73.5 ± 53.3 % | 76.5 ± 38.4% | 0.8 |
| Post-recon, lateral | 28.4 ± 20.6% | 23.01 ± 16.0% | 40.7 ± 24.8% | 0.003 |
| Post-NSM, medial | 63.3 ± 31.3% | 62.5 ± 30.8% | 65.2 ± 33.2% | 0.6 |
| Post-recon, medial | 29.2 ± 18.4% | 28.0 ± 23.3% | 32.1 ± 20.7% | 0.3 |
| Complications by Incision Type | ||||
| All patients (n=79) | IMF (n=55) | LR (n=24) | P | |
| Any Necrosis | 21 (26.5%) | 16 (29.1%) | 5 (20.8%) | 0.2 |
| Nipple tip necrosis | 16 (20.2%) | 13 (23.6%) | 3 (12.5%) | 0.5 |
| Nipple loss | 1 (1.3%) | 1 (1.8%) | 0 | 0.1 |
| Flap necrosis | 8 (10.1%) | 6 (10.9%) | 2 (8.3%) | 0.95 |
| Infection | 8 (10.1%) | 6 (10.9%) | 2 (8.3%) | 0.5 |
| Hematoma | 3 (3.8%) | 3 (5.5%) | 0 | 0.6 |
| Seroma | 5 (6.3%) | 4 (7.3%) | 1 (4.2%) | 1 |
| Device malposition | 6 (7.6%) | 5 (9.1%) | 1 (4.2%) | 0.7 |
| Implant exposure | 0 | 0 | 0 | 1 |
| Wound dehiscence | 2 (2.5%) | 1 (1.8%) | 1 (4.2%) | 0.5 |
| Total OR trips | 1.6 ± 0.7 | 1.6 ± 0.8 | 1.6 ± 0.6 | 0.9 |
| Explant | 4 (5.1%) | 4 (7.3%) | 0 | 0.3 |
Values presented as a percentage of the original preoperative fluorescent intensity, minus background fluorescence, based on 0-255 unit, 8-bit greyscale.
The rate of perfusion (FI greyscale units/second) did not differ significantly between incision types before or after mastectomy or reconstruction. The rate of perfusion to the nipple was most profoundly impacted by NSM (Figure 3) dropping 89.2% from pre-NSM to post-NSM, significantly more than the 38.1% (p<0.001) drop in FI greyscale units/second to the breast overall (Figure 3). The rate of perfusion to the inferior region was also significantly impacted by NSM (p=0.04) relative to the breast overall (Figure 3). By contrast, after reconstruction, the overall rate of perfusion to the breast dropped an additional 36.9%, but only minimally impacted the nipple (3% drop, p<0.001). The rate of perfusion to the lateral breast dropped an additional 52.2% (Figure 3), significantly more than the drop in overall breast perfusion (p=0.04).
Predictors of Perfusion
Covariates with an impact on perfusion are summarized in Table 5. Smoking (12.5% decrease, p=0.02) and MAP (1.5% decrease for every 5mmHg decrease in MAP, p=0.04) below the group mean MAP of 73.4 mmHg significantly impacted FI to the breast. An IMF incision predicted decreased FI in the lateral (17.5% decrease, p=0.001) and inferior regions (15% decrease, p=0.003). Decreased FI to the nipple was predicted by smoking (13% decrease, p=0.03) and larger specimen weight (3.4% decrease for every 100 gram increase over median, p=0.02). Reconstructed left breasts had lower FI rates than reconstructed right breasts (p=0.02).
Table 5.
Summary of covariates that had a statistically significant impact on skin and nipple fluorescent intensity and tissue necrosis.*
| Covariate | Impact | p-value |
|---|---|---|
| Perfusion – Skin Flap | ||
| Smoking | ↓ 12.5% | 0.02 |
| MAP | ↓ 1.5% for every 5 mmHg | 0.04 |
| IMF incision | ↓ 17.5% | 0.001 |
| Perfusion - Nipple | ||
| Smoking | ↓ 13% | 0.03 |
| Specimen weight | 3.4% for every 100 gram increase | 0.02 |
| Tissue Necrosis | ||
| Operative time | OR 1.1 per additional 10 minutess | 0.03 |
| Whole breast fluorescent intensity | OR 2.0 per 10% decrease in perfusion | 0.007 |
| Smoking | OR 6.7 for smokers | 0.03 |
| KPS | OR 4.5 for every 10 unit decrease | 0.01 |
The multivariate model for fluorescent intensity included smoking, age, specimen weight, laterality, MAP, incision, and operative time. For necrosis it included fluorescent intensity, smoking, age, specimen weight, mean arterial pressure during surgery, laterality, incision type and operative time.
Complications
Predictors of complications are summarized in Table 6. No differences in complication rates were found between incision types when adjusting for covariates. When necrosis occurred, average remaining nipple FI after reconstruction was 7.9% of preoperative FI, but 30.2% when there was no postoperative necrosis (p<0.01). Findings were similar for the inferior skin flap (13.3% vs 28.0%, respectively, p=0.01). Remaining FI of ≤11.8% to the nipple was 100% sensitive for necrosis, while remaining FI of ≤10.6% to the inferior skin flap was 100% sensitive for flap necrosis.
Table 6.
Predictors of necrosis and explantation following nipple-sparing mastectomy.
| NECROSIS | EXPLANTATION | |||
|---|---|---|---|---|
| ANY | NIPPLE | SKIN | ||
| Operating Room Time | OR 1.1 (95% CI 1.07, 1.18), per additional 10 min over median, p=0.03 | OR 1.1 (95% CI 1.03, 1.21) per additional 10 min over median, p=0.04 | ||
| Smoking | OR 6.7, p=0.03 (95% CI 3.6,8.9) | OR 1.4, p=0.04 (95% CI 1.15,2.3) |
OR 1.4, p =0.03 (95% CI 1.21,1.98) |
|
| Residual Fluorescent Intensity after Reconstruction | OR 2.0 for every 10% decrease, p=0.007 (95% CI 1.92,2.21) |
OR 2.1 for every 10% decrease, p=0.03 (95% CI 1.86,2.67) |
OR 1.8 for every 10% decrease, p=0.04 (95% CI 1.24,2.87) |
|
| Karnofsky Performance Score | OR 4.5 for every 10 unit decrease (95% CI 3.87, 5.86) |
|||
| Mean Arterial Pressure | OR 2.7 for every 5 mmHg decrease, p=0.03 (95% CI 2.13, 3.76) |
|||
| Body Mass Index | OR 1.4 for every 1 kg/m2 increase over 26 (p=0.04) (95% CI 1.12, 1.76) |
|||
Intramammary Compliance by Applanation Tonometry
Change in pre- and postoperative intramammary compliance was not associated with remaining FI to the breast, nipple, or flap necrosis. However, post-reconstruction intramammary compliance, as measured by tonometry, (Table 2) was significantly higher in the IMF group when compared to the LR group (1718.7 N/m2 vs 1164 N/m2, p=0.01).
Three-dimensional imaging
Preoperatively, the average breast volume, estimated by mammometrics, was 339±167 cc, while average breast specimen weight was 371±157 grams (See Table, Supplemental Digital Content 2, which shows Perioperative Q-scores as a function of incision selection, INSERT HYPER LINK). Breast volume, sternal notch-nipple, nipple-inframammary fold, and inter-nipple distance did not differ between the IMF and LR groups, although breast base width did (14.9cm vs 15.9cm, p=0.02). The absolute (p=0.01) and relative (p=0.01) increase in final post-operative volume was significantly higher in the IMF group based on differences in specimen weight and final implant size. The IMF group had a greater increase in nipple-inframammary fold distance relative to the LR group from pre- to postoperatively (1.5cm vs 0.9cm, p=0.04), but a smaller change in base width (−0.2cm vs −0.9 cm, p=0.03). The IMF group had implants with an overall larger base width, as well as a taller implant height and greater projection (Table 2).
Patient Reported Outcomes
Preoperatively (Table 7), “satisfaction with breasts” was higher for patients who received an IMF rather than a LR incision (p = 0.05). Preoperative “satisfaction with breasts” in patients chosing an IMF incision (63.8), versus those randomly assigned (61.2) was not significantly different. Postoperatively, patients with a LR incision reported a significantly greater improvement over their preoperative responses in “satisfaction with breasts” (16.5 vs 6.4, p=0.01) and “psychosocial wellbeing” (16.8 vs 6.4, p=0.04).
Table 7.
Patient Reported Outcomes by Incision Type.
| QScore by INCISION TYPE | |||||
|---|---|---|---|---|---|
| Preoperative | |||||
| IMF (n=49) | SD | LR (n=22) | SD | p | |
| Sat w/Breasts | 62.1 | 17.3 | 53.8 | 13.1 | 0.05 |
| Psychosocial | 74.1 | 16.5 | 68.0 | 12.9 | 0.1 |
| Phys chest | 88.3 | 14.0 | 85.7 | 12.2 | 0.4 |
| Sexuality | 59.4 | 10.3 | 56.7 | 10.8 | 0.5 |
| Postoperative | |||||
| Sat w/Breasts | 69.3 | 21.8 | 68.7 | 12.0 | 0.9 |
| Sat w/Outcome | 77.5 | 19.7 | 81.1 | 16.3 | 0.5 |
| Psychosocial | 79.8 | 19.1 | 85.2 | 17.0 | 0.2 |
| Sexuality | 68.7 | 25.3 | 68.6 | 17.2 | 1.0 |
| Phys Chest | 77.9 | 12.4 | 83.4 | 12.4 | 0.2 |
| Information | 87.8 | 14.1 | 80.6 | 14.4 | 0.06 |
| Surgeon | 97.8 | 7.4 | 95.9 | 8.2 | 0.4 |
| Medical staff | 98.0 | 8.7 | 89.1 | 23.0 | 0.02 |
| Office staff | 98.9 | 4.5 | 98.0 | 4.9 | 0.5 |
| Difference | |||||
| Sat w/Breasts | 6.7 | 25.8 | 15.8 | 14.2 | 0.1 |
| Psychosocial | 6.8 | 19.9 | 16.8 | 12.3 | 0.04 |
| Phys chest | −10.9 | 17.1 | −3.6 | 14.6 | 0.1 |
| Sexuality | 9.4 | 23.1 | 11.8 | 20.7 | 0.7 |
| QScore by Total # Procedures adj for preoperative Qscore | |||||
| 1 (n=42) | 2 (n=32) | 3 (n=2) | 4 (n=3) | p | |
| Sat w/Breasts | 70.1 | 71.9 | 63.1 | 39.2 | 0.04 |
| Sat w/Outcome | 87.3 | 81.2 | 75.0 | 74.0 | 0.8 |
| Psychosocial | 81.8 | 83.5 | 77.5 | 61.6 | 0.02 |
| Sexuality | 70.5 | 69.0 | 72.0 | 29.6 | 0.04 |
| Phys Chest | 81.7 | 77.6 | 58.6 | 88.0 | 0.2 |
| Information | 85.4 | 85.8 | 87.0 | 86.7 | 1.0 |
| Surgeon | 96.3 | 98.5 | 95.5 | 100.0 | 0.6 |
| Medical staff | 97.2 | 92.0 | 100.0 | 100.0 | 0.5 |
| Office staff | 98.8 | 98.1 | 100.0 | 100.0 | 0.8 |
Implant explantation contributed to a decrease in “satisfaction with breasts” (−27.4, p<0.01), “satisfaction with outcomes” (−18.6, p=0.05), and “psychosocial well-being” (38.4, p<0.01). Explantation and infection decreased “sexual well-being” scores by 40.7 (p<0.01) and 12.8 (p=0.01), respectively. Hematoma and explantation led to decreased “physical well-being” of the chest lowering scores 16.0 (p=0.03) and 11.1 (p=0.05), respectively. All values were adjusted for preoperative scores when applicable. An increasing number of reconstructive procedures adversely affected “satisfaction with breasts”, “psychosocial well-being”, and “sexual well-being” (Table 7).
DISCUSSION
In this study, we demonstrated that incision selection can impact both clinical and patient-reported outcomes following NSM with prosthetic breast reconstruction. Preoperative perfusion pattern did not impact postoperative perfusion, as measured by FI, or complications (Table 3). While we hypothesized that an IMF incision would be least disruptive to the blood supply, perfusion to the inferior and lateral skin flaps diminished more after IMF than LR incisions (Table 4). In addition, the upper pole of the breast can be difficult to access through an IMF incision particularly in individuals with a long torso, or large breast, thus raising concerns about the adequacy of oncologic resection.(31) We utilized an IMF incision 11.3±1.9 cm long, adequate for optimizing visualization,(29) and yet we observed hypoperfusion in the inferolateral breast consistent with surgical retraction in several patients with IMF incisions (Fig 2D). Overall perfusion to the breast was substantively impacted by placement of a breast prosthesis, with the rate of change in FI dropping an additional 36.9% relative to post-NSM levels. These data confirm that while NSM compromises blood supply to the breast skin, the decision to proceed with either a tissue expander or immediate implant, and select an appropriate volume can have measureable impact on skin flap perfusion. Interestingly, the rate of perfusion was significantly slower in left versus right breasts. For a right handed surgeon, visualization of the superolateral axillary tail and sentinel lymph node of the left breast through either an IMF or LR incision often affords more limited sight-lines compared to the right side. Subjectively we have noted that this leads to increased pressure of retraction on the left side to optimize visualization, potentially compromising perfusion.
Despite the reduction in perfusion to the inferior and lateral mastectomy skin flaps following IMF incision, complication rates following NSM and reconstruction did not differ between IMF and LR groups. Thin mastectomy flaps that disrupt cutaneous perforators coursing 1cm below the skin surface may have a greater impact on perfusion-related complications than incision selection.(32) This discrepancy may be associated with the fact that tissue viability assumes a binary, viable or not, rather than a linear relationship with perfusion and subsequent complications.(33) Regardless of etiology, reduction of mastectomy flap perfusion to 10.6%, or nipple perfusion to 11.8%, of its original rate was uniformly associated with necrosis. Laser-assisted angiography can overpredict hypoperfusion and may have also impacted the correlation between perfusion and complications.(34) Complication rates in our series were consistent with other reports,(24, 35-39) and were predicted by smoking, operative time, specimen weight, a lower KPS, and MAP. Increases in operative time were noted in breasts of greater surface area and volume. The resection of larger volumes of tissue is both time consuming, but also more challenging, as described several times for NSM.(29, 31, 40) Smoking and an elevated BMI are widely known to increase complication rates with NSM and their impact on reconstructive failure should be accounted for.(41-43) Delaying high risk cases in patients with larger breasts, higher BMI, or in patients who are smokers may minimize risks, as delaying implant-based reconstruction has been shown to independently decrease the risk of complications.(44-47) The impact of intraoperative MAP on complication rates in NSM is a unique finding and underscores the impact of perfusion pressure on the mastectomy skin flaps regardless of cutaneous perforator anatomy. Based on this finding, we now counsel anesthesiologists to maintain a MAP above 70 mmHg.
Patient satisfaction with breasts, psychosocial well-being and sexual well-being were all significantly improved 3 months following NSM with implant reconstruction, consistent with some studies,(11, 13) although others have reported less favorable outcomes due to limited recovery of nipple sensibility.(12, 48) Consistent with previous work, physical well-being dropped off after NSM.(12) Despite improvements in aesthetic outcomes, pain,(49) animation deformity,(50) and depression may all adversely impact physical well-being following NSM.(51) Enhanced recovery after surgery protocols to limit pain,(52) pre-pectoral reconstructions to limit chest wall compromise,(53) and self-efficacy strategies that optimize coping represent important targets for improving physical well being and overall outcomes.(54)
Subtle differences in health-related quality of life were noted in the IMF versus LR incision groups. Preoperatively, satisfaction with breasts was higher in the IMF group versus the LR incision group (p=0.05). In cases where patients demonstrated preference, 84% chose the IMF incision over the LR incision (See Figure, Supplemental Digital Content 1, INSERT HYPER LINK). Satisfaction with breasts, however, did not significantly differ when patients chose (63.8), or were randomized (61.2) to, the IMF group. Moreover, postoperatively, “psychosocial well-being” improved significantly more in the LR than the IMF group (p=0.04). Intramammary compliance was significantly increased in the IMF cohort (p=0.01), a direct result of placing relatively larger breast implants into the soft tissue pocket as measured by breast surface area (Table 2).
To adequately remove breast tissue, including the upper pole and sentinel lymph node through a single incision, mean incision length was significantly longer in the IMF than the LR group (p<0.001). A longer scar may have adversely impacted psychosocial well-being, satisfaction with breasts, and physical well-being of the chest. Also, patients with an IMF incision ultimately received a significantly larger breast implant with greater height, projection, and base width than patients with a LR incision despite equivalent breast volume, and surface area preoperatively and specimen weight intraoperatively (Table 2). Predictably, breast volume and nipple-inframammary fold distances increased signficiantly more in the IMF than LR incision groups (See Table, Supplemental Digital Content 2, INSERT HYPER LINK) since the inframammary fold needed to be dropped a greater distance with larger implants to centralize the nipple-areola complex. Importantly, an increased inframammary fold to nipple distance was not associated with nipple-areola malposition in any cases in this series. Absence of nipple-areola malposition in this series may be due to our exclusion of patients with vertical radial incisions or autologous reconstructions which are associated with this deformity (35).
While IMF incisions appeared to elongate the height of the breast, probably due to a relative increase in implant volume, LR incisions appeared to narrow the width of the breast (See Table, Supplemental Digital Content 2, INSERT HYPER LINK). Unlike mean implant height and projection, which was significantly higher in patients in the IMF cohort, implant base width did not differ significantly between incision cohorts. We suspect that this difference relates primarily to scar formation and surgical management of the skin edges. Following a LR incision, the lateral skin edges are compromised by surgical retraction leading to scar formation, potential resection of the incision margin following placement of a tissue expander, and excision of a widened scar at the time of implant exchange. Increased scar contracture and tension on the skin envelope may narrow the base width of implant reconstructions performed through a LR more than an IMF incision.
Incision type did not impact the overall number of procedures required to complete prosthetic reconstruction (Table 3). A single procedure was required in 53.2% of patients, while 40.5% required a second procedure either to exchange a tissue expander for an implant or to revise an immediate implant and perform fat grafting for contour correction (Table 7).(55) Postoperatively, satisfaction with breasts, and psychosocial- and sexual well-being fell in the small group (3.8%) who required a fourth procedure (Table 7). With this limited number of cases it is impossible to say whether this decline in patient scores drove patients to have more procedures, or whether a greater number of procedures to manage complications or unacceptable aesthetic outcomes diminished PROs.
This study has several limitations. We measured FI to analyze blood flow, but perfusion is more complex and depends on factors like vascular tone, patency, temperature, and oxygen saturation. The Spy device lacks direct physiologic correlation and is also subject to physical factors like camera position and ambient light. While the two most commonly utilized incisions were analyzed, surgical oncologists also employ other incisions.(23, 40) Oncologic considerations, patient and surgeon preferences, pre-existing anatomy and scars all impact incision choice, potential outcomes, and the generalizability of our results.(29, 56, 57) These considerations precluded our ability to randomize this study and introduced selection bias that limited internal validity. In addition, we may not have waited long enough following placement of the permanent breast implant to sample PROs. However, whether compared to preoperative values or unadjusted, our Breast Q scores at 3 months compared favorably with other reports that included longer followup.(11, 12, 58)
CONCLUSIONS
Incision selection may impact skin perfusion, mammometrics, and PROs, but not complication rates, following NSM with breast reconstruction. Our findings provide detailed information on the impact of incision location in NSM that can help guide clinical decision-making for breast cancer patients. While immediate implant breast reconstruction has the obvious advantage of limiting the number of reconstructive procedures, our perfusion data clearly shows that not only NSM, but reconstruction itself increases tension on the skin flaps mandating careful attention to alterations in perfusion, and implant volume selection. During preoperative consultation, most patients in our study preferred the IMF incision and this remains the favored option in our practice for implant-based post-NSM breast reconstruction. Our PRO and perfusion data, however, suggest that the LR incision can also produce favorable results. We favor the LR incision for post-NSM microvascular breast reconstruction based on improved access to the internal mammary arteries.
Supplementary Material
Figure, Supplemental Digital Content 1. Of 59 patients in the non-randomized cohort, 38 patients had an IMF incision. This was based on patient preference in 27 cases (71% of non-randomized IMF incision cases) and oncologic surgeon bias based on pre-existing scars or oncologic reasons in 11 cases (29%). The LR incision was deliberately selected by patients in 5 cases (56% if non-randomized LR incision cases), and by the oncologic surgeon in the remainder. In 32 cases where incision selection was patient-driven and randomization was refused, patients selected the IMF incision over the LR incision in 84% of cases, INSERT HYPER LINK.
Table, Supplemental Digital Content 2. Perioperative Q-scores as a function of incision selection, INSERT HYPER LINK.
Acknowledgments
The authors would like to thank from the Department of Surgery at Washington University School of Medicine in Saint Louis, Ms. Colleen Kilbourne-Glynn for assistance with patient enrollment and data organization. They would like to thank Ms. Annette Irving for assistance with IRB preparation and Ms. Tracey Guthrie for administrative assistance.
Financial Disclosure:
Dr. Myckatyn is a consultant for Acelity and Allergan. Acelity initially provided Spy kits for perfusion assessment to Dr. Tenenbaum for this study. No other funds or resources were provided for this study. None of the other authors have relevant financial disclosures.
Footnotes
Meeting Presentation: These data were presented in part at Plastic Surgery The Meeting, American Society of Plastic Surgeons, October 2016, Los Angeles, California, USA
References
- 1.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 2.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 3.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg. 2017;265:581–589. doi: 10.1097/SLA.0000000000001698. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Park EH, Lim WS, et al. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Annals of Surgery. 2010;251:493–498. doi: 10.1097/SLA.0b013e3181c5dc4e. [DOI] [PubMed] [Google Scholar]
- 5.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 6.Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238:120–127. doi: 10.1097/01.SLA.0000077922.38307.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 8.Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg. 2013;131:969–984. doi: 10.1097/PRS.0b013e3182865a3c. [DOI] [PubMed] [Google Scholar]
- 9.Spear SL, Hannan CM, Willey SC, Cocilovo C. Nipple-sparing mastectomy. Plast Reconstr Surg. 2009;123:1665–1673. doi: 10.1097/PRS.0b013e3181a64d94. [DOI] [PubMed] [Google Scholar]
- 10.Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol. 2009;27:4948–4954. doi: 10.1200/JCO.2008.20.8785. [DOI] [PubMed] [Google Scholar]
- 11.Howard MA, Sisco M, Yao K, et al. Patient satisfaction with nipple-sparing mastectomy: A prospective study of patient reported outcomes using the BREAST-Q. J Surg Oncol. 2016;114:416–422. doi: 10.1002/jso.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peled AW, Duralde E, Foster RD, et al. Patient-reported outcomes and satisfaction after total skin-sparing mastectomy and immediate expander-implant reconstruction. Ann Plast Surg. 2014;72(Suppl 1):S48–52. doi: 10.1097/SAP.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe KA, Cil TD, Semple JL, et al. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann Surg Oncol. 2015;22:3324–3330. doi: 10.1245/s10434-015-4761-3. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. British Journal of Plastic Surgery. 1987;40:113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 15.Acheson A, Lindsay RM. Non target-derived roles of the neurotrophins. Philos Trans R Soc Lond B Biol Sci. 1996;351:417–422. doi: 10.1098/rstb.1996.0037. [DOI] [PubMed] [Google Scholar]
- 16.Taylor GI, Caddy CM, Watterson PA, Crock JG. The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg. 1990;86:185–213. doi: 10.1097/00006534-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lista F, Ahmad J. Vertical scar reduction mammaplasty: a 15-year experience including a review of 250 consecutive cases. Plastic and Reconstructive Surgery. 2006;117:2152–2165. doi: 10.1097/01.prs.0000218173.16272.6c. discussion 2166-2159. [DOI] [PubMed] [Google Scholar]
- 18.van Deventer PV, Graewe FR. The Blood Supply of the Breast Revisited. Plast Reconstr Surg. 2016;137:1388–1397. doi: 10.1097/PRS.0000000000002048. [DOI] [PubMed] [Google Scholar]
- 19.Hall-Findlay EJ. Discussion: The Blood Supply of the Breast Revisited. Plast Reconstr Surg. 2016;137:1398–1400. doi: 10.1097/PRS.0000000000002123. [DOI] [PubMed] [Google Scholar]
- 20.Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol. 2014;21:100–106. doi: 10.1245/s10434-013-3214-0. [DOI] [PubMed] [Google Scholar]
- 21.Swistel A, Small K, Dent B, Cohen O, Devgan L, Talmor M. A novel technique of preserving internal mammary artery perforators in nipple sparing breast reconstruction. Plastic and reconstructive surgery Global open. 2014;2:e198. doi: 10.1097/GOX.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962;30:676–682. doi: 10.1097/00006534-196212000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054. doi: 10.1097/PRS.0b013e3182a48b8a. [DOI] [PubMed] [Google Scholar]
- 24.de Alcantara Filho P, Capko D, Barry JM, Morrow M, Pusic A, Sacchini VS. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the memorial sloan-kettering cancer center experience. Annals of surgical oncology. 2011;18:3117–3122. doi: 10.1245/s10434-011-1974-y. [DOI] [PubMed] [Google Scholar]
- 25.Ashikari RH, Ashikari AY, Kelemen PR, Salzberg CA. Subcutaneous mastectomy and immediate reconstruction for prevention of breast cancer for high-risk patients. Breast Cancer. 2008;15:185–191. doi: 10.1007/s12282-008-0059-7. [DOI] [PubMed] [Google Scholar]
- 26.Salzberg CA, Ashikari AY, Koch RM, Chabner-Thompson E. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm) Plastic and Reconstructive Surgery. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 27.Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Annals of Plastic Surgery. 2012;68:446–450. doi: 10.1097/SAP.0b013e3182394bba. [DOI] [PubMed] [Google Scholar]
- 28.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506. doi: 10.1097/01.prs.0000438056.67375.75. [DOI] [PubMed] [Google Scholar]
- 29.Wijayanayagam A, Kumar AS, Foster RD, Esserman LJ. Optimizing the total skin-sparing mastectomy. Arch Surg. 2008;143:38–45. doi: 10.1001/archsurg.143.1.38. discussion 45. [DOI] [PubMed] [Google Scholar]
- 30.Karnofsky D, Abelmann W, Craver L, Burchenal J. The Use of the Nitrogen Mustards in the Palliative Treatment of Carcinoma - with Particular Reference to Bronchogenic Carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 31.Chen CM, Disa JJ, Sacchini V, et al. Nipple-sparing mastectomy and immediate tissue expander/implant breast reconstruction. Plast Reconstr Surg. 2009;124:1772–1780. doi: 10.1097/PRS.0b013e3181bd05fd. [DOI] [PubMed] [Google Scholar]
- 32.Michelle le Roux C, Kiil BJ, Pan WR, Rozen WM, Ashton MW. Preserving the neurovascular supply in the Hall-Findlay superomedial pedicle breast reduction: an anatomical study. J Plast Reconstr Aesthet Surg. 2010;63:655–662. doi: 10.1016/j.bjps.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Munabi NC, Olorunnipa OB, Goltsman D, Rohde CH, Ascherman JA. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67:449–455. doi: 10.1016/j.bjps.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Mattison GL, Lewis PG, Gupta SC, Kim HY. SPY Imaging Use in Postmastectomy Breast Reconstruction Patients: Preventative or Overly Conservative? Plast Reconstr Surg. 2016;138:15e–21e. doi: 10.1097/PRS.0000000000002266. [DOI] [PubMed] [Google Scholar]
- 35.Choi M, Frey JD, Salabian AA, Karp NS. Nipple-Areola Complex Malposition in Nipple-Sparing Mastectomy: A Review of Risk Factors and Corrective Techniques from Greater Than 1000 Reconstructions. Plast Reconstr Surg. 2017 doi: 10.1097/PRS.0000000000003507. [DOI] [PubMed] [Google Scholar]
- 36.Manning AT, Sacchini VS. Conservative mastectomies for breast cancer and risk-reducing surgery: the Memorial Sloan Kettering Cancer Center experience. Gland Surg. 2016;5:55–62. doi: 10.3978/j.issn.2227-684X.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diep GK, Hui JY, Marmor S, et al. Postmastectomy Reconstruction Outcomes After Intraoperative Evaluation with Indocyanine Green Angiography Versus Clinical Assessment. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5466-y. [DOI] [PubMed] [Google Scholar]
- 38.Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg. 2012;129:1043–1048. doi: 10.1097/PRS.0b013e31824a2b02. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Etienne CA, Cody HS, Iii 3rd, Disa JJ, Cordeiro P, Sacchini V. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. The breast journal. 2009;15:440–449. doi: 10.1111/j.1524-4741.2009.00758.x. [DOI] [PubMed] [Google Scholar]
- 40.Munhoz AM, Montag E, Filassi JR, Gemperli R. Immediate nipple-areola-sparing mastectomy reconstruction: An update on oncological and reconstruction techniques. World J Clin Oncol. 2014;5:478–494. doi: 10.5306/wjco.v5.i3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi AA, Broderick KP, Belz J, et al. Uneventful versus Successful Reconstruction and Outcome Pathways in Implant-Based Breast Reconstruction with Acellular Dermal Matrices. Plast Reconstr Surg. 2016;138:173e–183e. doi: 10.1097/PRS.0000000000002402. [DOI] [PubMed] [Google Scholar]
- 42.Quinn TT, Miller GS, Rostek M, Cabalag MS, Rozen WM, Hunter-Smith DJ. Prosthetic breast reconstruction: indications and update. Gland Surg. 2016;5:174–186. doi: 10.3978/j.issn.2227-684X.2015.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A. Complications analysis of 266 immediate breast reconstructions. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2006;59:1017–1024. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Davies K, Allan L, Roblin P, Ross D, Farhadi J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast. 2011;20:21–25. doi: 10.1016/j.breast.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Martinez CA, Reis SM, Boutros SG. The Nipple–Areola Preserving Mastectomy: The Value of Adding a Delay Procedure. Plastic and Reconstructive Surgery Global Open. 2016;4:e1098. doi: 10.1097/GOX.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinovic ME, Pellicane JV, Blanchet NP. Surgical Delay of the Nipple–Areolar Complex in High-risk Nipple-sparing Mastectomy Reconstruction. Plastic and Reconstructive Surgery Global Open. 2016;4:e760. doi: 10.1097/GOX.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 48.van Verschuer VM, Mureau MA, Gopie JP, et al. Patient Satisfaction and Nipple-Areola Sensitivity After Bilateral Prophylactic Mastectomy and Immediate Implant Breast Reconstruction in a High Breast Cancer Risk Population: Nipple-Sparing Mastectomy Versus Skin-Sparing Mastectomy. Ann Plast Surg. 2016;77:145–152. doi: 10.1097/SAP.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 49.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32:1703–1711. doi: 10.1200/JCO.2013.54.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammond DC, Schmitt WP, O’Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg. 2015;135:1540–1544. doi: 10.1097/PRS.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 51.Rief W, Bardwell WA, Dimsdale JE, Natarajan L, Flatt SW, Pierce JP. Long-term course of pain in breast cancer survivors: a 4-year longitudinal study. Breast Cancer Res Treat. 2011;130:579–586. doi: 10.1007/s10549-011-1614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temple-Oberle C, Shea-Budgell MA, Tan M, et al. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg. 2017;139:1056e–1071e. doi: 10.1097/PRS.0000000000003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg. 2017;139:287–294. doi: 10.1097/PRS.0000000000002950. [DOI] [PubMed] [Google Scholar]
- 54.Sheinfeld Gorin S, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30:539–547. doi: 10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi AA, Odom EB, Parikh RP, Myckatyn TM, Tenenbaum MM. Patient-Reported Outcomes of Aesthetics and Satisfaction in Immediate Breast Reconstruction After Nipple-Sparing Mastectomy With Implants and Fat Grafting. Aesthet Surg J. 2017 doi: 10.1093/asj/sjx048. [DOI] [PubMed] [Google Scholar]
- 56.Stolier AJ, Sullivan SK, Dellacroce FJ. Technical considerations in nipple-sparing mastectomy: 82 consecutive cases without necrosis. Ann Surg Oncol. 2008;15:1341–1347. doi: 10.1245/s10434-007-9753-5. [DOI] [PubMed] [Google Scholar]
- 57.Colwell AS, Gadd M, Smith BL, Austen WG., Jr An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Annals of Plastic Surgery. 2010;65:140–143. doi: 10.1097/SAP.0b013e3181c1fe77. [DOI] [PubMed] [Google Scholar]
- 58.Weichman KE, Hamill JB, Kim HM, Chen X, Wilkins EG, Pusic AL. Understanding the recovery phase of breast reconstructions: Patient-reported outcomes correlated to the type and timing of reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:1370–1378. doi: 10.1016/j.bjps.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 1. Of 59 patients in the non-randomized cohort, 38 patients had an IMF incision. This was based on patient preference in 27 cases (71% of non-randomized IMF incision cases) and oncologic surgeon bias based on pre-existing scars or oncologic reasons in 11 cases (29%). The LR incision was deliberately selected by patients in 5 cases (56% if non-randomized LR incision cases), and by the oncologic surgeon in the remainder. In 32 cases where incision selection was patient-driven and randomization was refused, patients selected the IMF incision over the LR incision in 84% of cases, INSERT HYPER LINK.
Table, Supplemental Digital Content 2. Perioperative Q-scores as a function of incision selection, INSERT HYPER LINK.


