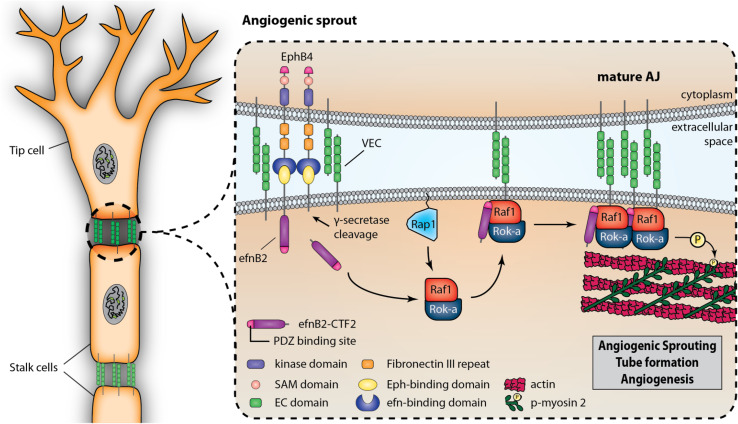
Fig. 8.
PS1/γ-secretase promotes EphB4-induced sprouting via processing of efnB2. EphB4 binding to efnB2 induces cleavage of the latter by PS1/γ-secretase, producing cytoplasmic peptide efnB2/CTF2. This peptide acts as a scaffolding protein, promoting the assembly of angiogenic complexes including VE-cadherin, Raf-1 and Rok-α in a Rap1-dependent manner and increasing phosphorylation of MLC2 at VE-cadherin-containing cell–cell contact sites. Peptide efnB2/CTF2 potently increases endothelial cell sprouting and tube formation and all above functions are mediated by its PDZ-binding domain. All functions are inhibited by downregulation of PS1 or presence of γ-secretase inhibitors