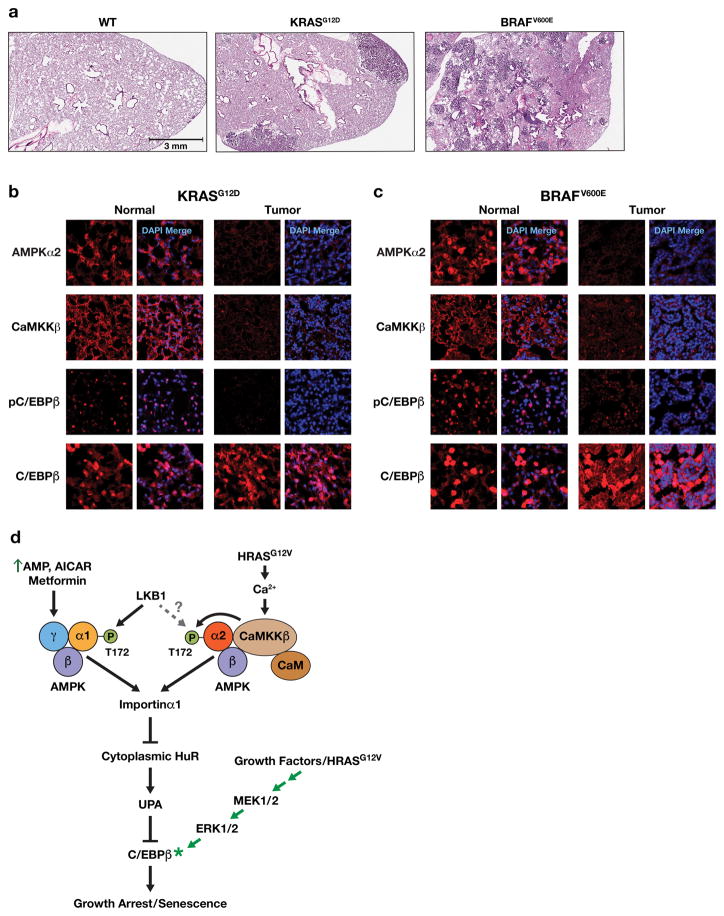
Figure 8.
AMPKα2, CaMKKβ and p-C/EBPβ levels are markedly decreased in KRASG12D- and BRAFV600E-driven mouse lung tumors. (a) H&E stained lung areas from a normal WT mouse, a KrasLA2/+ mouse45 containing adenocarcinomas (ADC) and a LSL-BRAFV600E/+ animal46 bearing multiple adenomas. (b) Normal and lung tumor areas from a 165 day-old KrasLA2/+ mouse; sections were immunostained for AMPKα2, CaMKKβ, p-C/EBPβ (Thr188) and total C/EBPβ. (c) Normal and lung tumor areas from a 190 day-old LSL-BRAFV600E/+ animal (103 days after intratracheal instillation of Ad.Cre virus); sections were immunostained for AMPKα2, CaMKKβ, p-C/EBPβ (Thr188) and total C/EBPβ. (d) Model depicting AMPK-dependent pathways that mediate C/EBPβ activation and senescence in response to energy stress/AMPK agonists or oncogenic RAS. AMPK signaling suppresses the UPA mechanism that inhibits C/EBPβ activation and thus licenses C/EBPβ activation. To become activated, C/EBPβ also requires signaling through the RAS-ERK cascade to induce phosphorylation on Thr188 as well as other modifications5,62.