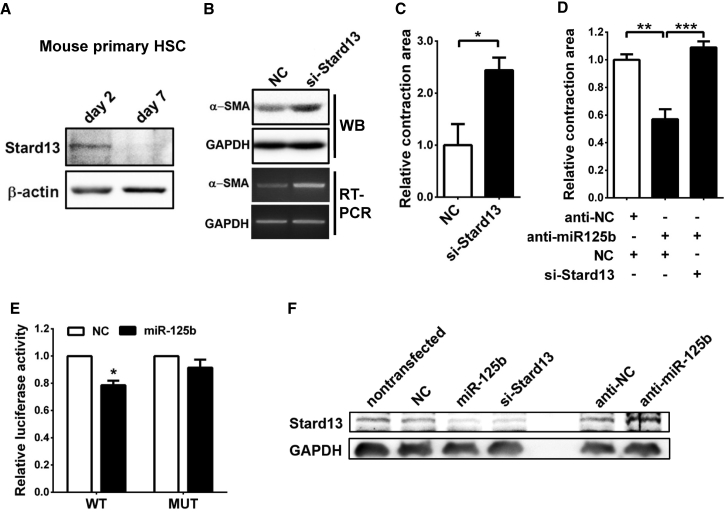
Figure 5.
miR-125b Suppresses the Expression of Stard13 by Directly Binding to its 3′ UTR
(A) The protein level of Stard13 was decreased in the culture-activated HSCs. Mouse primary HSCs were cultured in vitro for 2 or 7 days before western blotting. (B) Knockdown of Stard13 promoted α-SMA expression. JS1 cells were transfected with NC or si-Stard13 for 48 hr, followed by western blotting or RT-PCR analysis. (C) Inhibition of Stard13 enhanced cell contraction. (D) Silencing of Stard13 attenuated the suppressive effect of anti-miR-125b on JS1 contraction. For (C) and (D), the collagen lattice, which contained JS1 cells transfected with the indicated siRNA (50 nM) or anti-miR-125b (50 nM), was released and then incubated with 10% FBS-containing DMEM for 24 hr before measuring contraction area. (E) Overexpression of miR-125b suppressed the activity of luciferase reporter containing wild-type, but not mutant, 3′ UTR of Stard13. JS1 cells were cotransfected with NC or miR-125b duplexes and luciferase reporter plasmids that contained either wild-type (WT) or mutant (MUT) 3′ UTR of Stard13 for 48 hr before luciferase assay. The Renilla luciferase activity of each sample was normalized to that of firefly luciferase. (F) Overexpression of miR-125b reduced cellular Stard13 level, and knockdown of endogenous miR-125b increased Stard13 expression. JS1 cells without transfection (lane 1) or transfected with the indicated RNA were incubated for 48 hr before western blotting analysis. Data are presented as mean ± SEM in (E)–(E). *p < 0.05; **p < 0.01; ***p < 0.001.