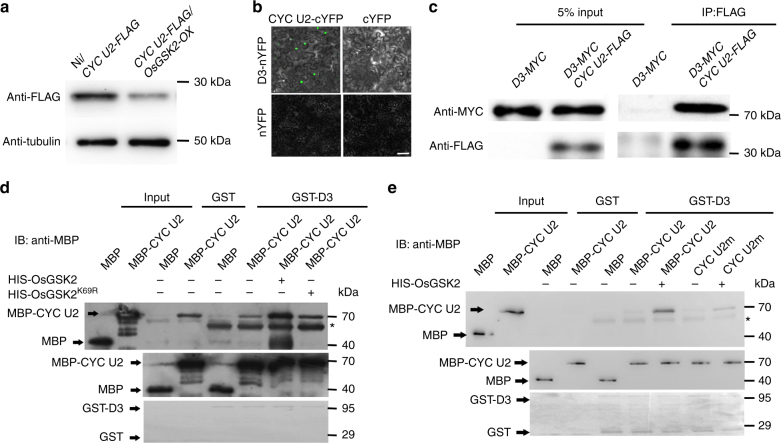
Fig. 4.
The OsGSK2-phosphorylated CYC U2 interacts with D3. a CYC U2 protein levels in the Ni/CYC U2-FLAG and CYC U2-FLAG/OsGSK2-OX F1 hybrids lines. The wild-type Nipponbare crossed with CYC U2-FLAG as a control. The mesocotyls are pre-treated with cycloheximide (CHX) for 3 h. Anti-FLAG was used to detect CYC U2-FLAG fusion protein level, and anti-tubulin was used for equal loading. b Interactions between CYC U2 and D3 in BiFC assays. Scale bar, 100 µm. c Interaction between CYC U2 and D3 in the Co-IP assays. The proteins were expressed and extracted from tobacco leaf and immunoprecipitated by anti-FLAG M2 magnetic beads. Gel blots were probed with anti-FLAG or anti-MYC antibody. d D3 interacts with the OsGSK2-phosphorylated CYC U2 in GST pull-down assays. The kinase dead type, OsGSK2K69R-HIS, is as the negative control to OsGSK2-HIS. MBP-CYC U2 was preincubated with OsGSK2-HIS and OsGSK2K69R-HIS, respectively, and then subjected to the pull-down assay. The top panel is the result of pull-down assay immunoblot with anti-MBP; the bottom and middle panels are the protein loading control. Asterisk indicates the nonspecific binds. e The CYC U2m exhibits a reduced interaction with D3 in GST pull-down assay. Asterisk indicates the nonspecific binds