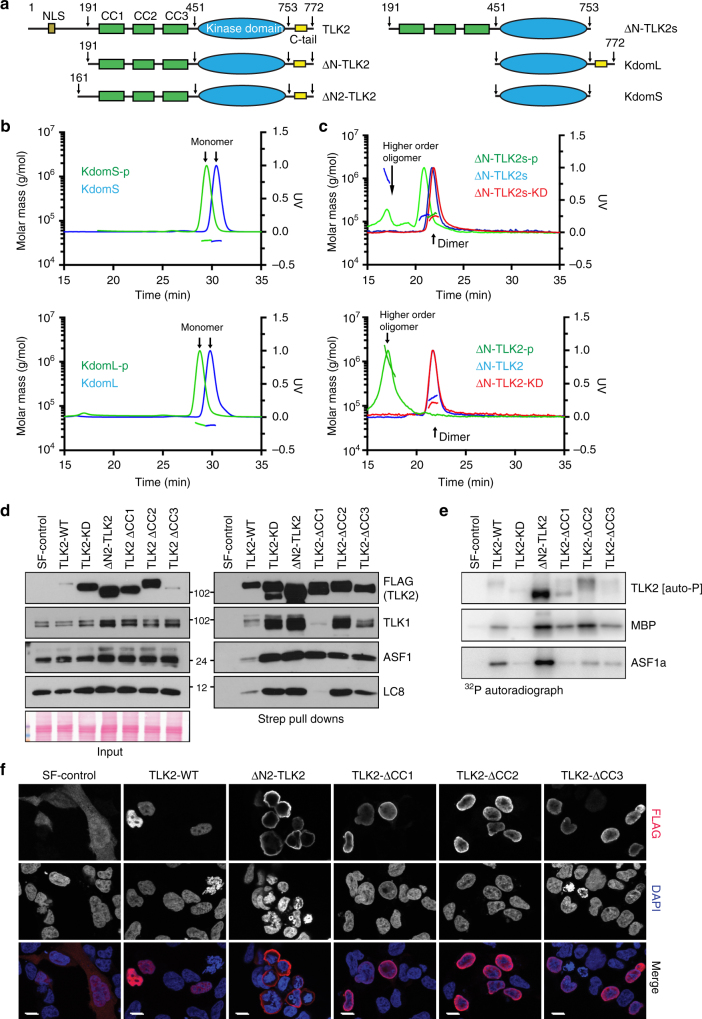
Fig. 1.
Architecture and characterization of TLK2. a TLK2 domain architecture and constructs used in this study. For a complete list see Supplementary Figure 2. b Size exclusion chromatography (SEC) coupled with Multi-Angle Laser Light Scattering (MALLS) profiles were used to assess the oligomerization state of the phosphorylated and unphosphorylated constructs with or without the C-tail for the kinase domain and c ΔN-TLK2 constructs. The effect of the catalytically inactive mutant ΔN-TLK-KD (D613A mutation) is also included. d The indicated TLK2 mutants were overexpressed in AD293 cells by transient transfection and pulled down from cell lysates using Streptavidin resin. Protein–protein interactions were analyzed by western blotting for TLK2, TLK1, ASF1 and LC8 (right panel). Input levels are shown in the left panel including the Ponceau red-stained membrane showing similar total protein levels. The KD in this experiment bears the D592V mutation instead of D613A. e Kinase assays were performed from Streptavidin pull-downs performed as in d. Kinase complexes were incubated with either MBP or ASF1a in the presence of 32P-ATP and autophosphorylation (TLK2) and substrate phosphorylation assessed in dried SDS-PAGE gels exposed to a phosphoimager. f Immunofluorescence analysis of exogenous TLK2 in transiently transfected AD293 cells. Localization of TLK2 and mutant forms was determined by staining with anti-FLAG antibodies and co-staining of nuclei with DAPI. Cytoplasmic localization of the ∆N-TLK2 mutant confirms the presence of the NLS in the N-terminus of the protein. The scale bar represents 10 μm in all the pictures. Uncropped gels and blots are shown in Supplementary Figures 10–15