Figure 2.
Identification of a Candidate Binding Domain to Target trans-Splicing Molecules to CEP290 Intron X-27
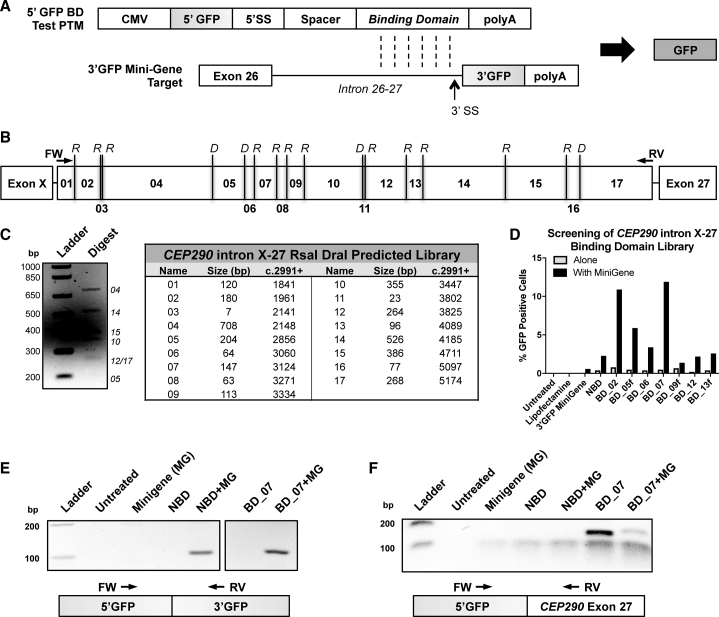
(A) Schematic of trans-splicing between a 5′ binding domain (BD) test PTM encoding the 5′ portion of GFP (5′ GFP) and a mini-gene target encoding the 3′ portion of GFP (3′ GFP). Watson-Crick base pairing is indicated by vertical dashed lines. Trans-splicing between the two pre-mRNAs results in reconstitution and expression of GFP. (B) Diagram of RsaI (R) and DraI (D) restriction sites within a region of CEP290 intron X-27. (C) Agarose gel electrophoresis after restriction enzyme digestion of a PCR fragment corresponding to the region described in (B). The fragment was amplified from genomic DNA, digested with restriction enzymes RsaI and DraI, and visualized on a 2% TBE-agarose gel. The fragment library numbers of visible bands of expected sizes are indicated according to the table of predicted fragments. (D) Quantitation by flow cytometry of GFP expression in HEK293T transiently transfected with plasmids encoding a fragment library test PTM (gray bars) or co-transfected with a test PTM and the 3′ GFP target (black bars). Samples with “f’ demark forward orientation that is not predicted to confer trans-splicing specificity; however, BD_05f did yield a slight improvement to GFP expression over no binding domain (NBD). (E) Agarose gel electrophoresis following RT-PCR using cDNA generated from HEK293T cells transfected with plasmids in (D). Primers were designed to specifically bind to the 5′ or 3′ portions of the GFP coding DNA sequence to validate trans-splicing between the 5′ test PTM and the 3′ GFP target pre-mRNAs. Data for the other test PTMs has been removed at the break indicated. Interestingly, untargeted PTM (NBD) also resulted in trans-splicing of RNA in agreement with observed GFP expression. MG, mini-gene. (F) Samples from (E) with primers designed to specifically bind to the 5′ portion of GFP or to exon 27 of Homo sapiens CEP290. Image has been contrast enhanced to visualize the faint band in lane 7 for co-transfection of BD_07 with target mini-gene (MG).