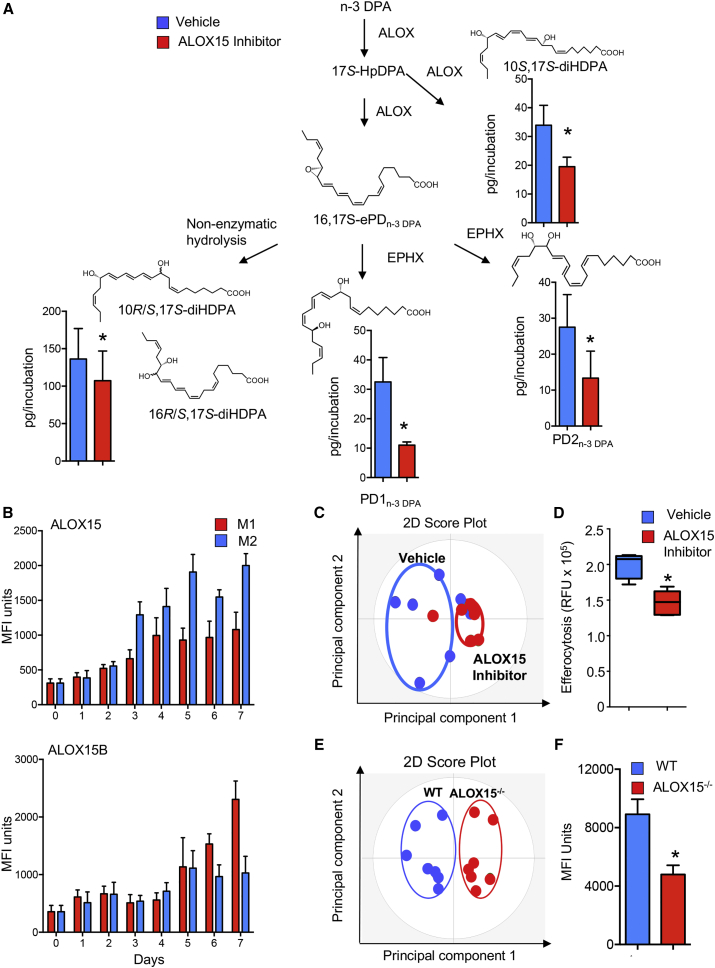
Figure 1.
Inhibiting 15-Lipoxygenase Activity Reduces PDn-3 DPA Production Dysregulating Macrophage Phenotype and Function
(A) Human monocytes were incubated with M-CSF (20 ng/mL) and either a ALOX15 inhibitor or vehicle (37°C, 5% CO2). On day 7 incubations were quenched, lipid mediators were extracted, identified, and quantified using lipid mediator profiling (see the STAR Methods for details). Results are mean ± SEM. n = 6 donors. *p < 0.05.
(B) Human monocytes were isolated and incubated with GM-CSF (20 ng/mL), IFN-γ (20 ng/mL), and LPS (100 ng/mL) to produce M1 or M-CSF (20 ng/mL) and IL-4 (20 ng/mL) to obtain M2 cells, and the expression of ALOX15 and ALOX15B was evaluated during the differentiation time course using flow cytometry. Results are mean ± SEM n = 4–6 donors per interval.
(C and D) Human monocytes were incubated with vehicle or ALOX15 inhibitor and then with M-CSF (20 ng/mL) for 7 days, and (C) expression of lineage markers was determined using fluorescently labeled antibodies and flow cytometry on day 7, and interrogated using OPLS-DA. n = 6 donors. (D) Phagocytosis of fluorescently labeled apoptotic cells investigated. Results for are mean ± SEM. n = 6 donors. *p < 0.05.
(E) Peritoneal macrophages were harvested from wild-type (WT) and ALOX15−/− mice, and the expression of lineage markers on CD64+ cells was determined using flow cytometry. Results were interrogated using OPLS-DA and are representative of n = 7 mice.
(F) Fluorescently labeled apoptotic cells were administered to WT and ALOX15−/− mice via intraperitoneal injection. After 1 hr peritoneal cells were harvested, and phagocytosis of apoptotic cells by CD64+ cells was evaluated using flow cytometry. Results are mean ± SEM. n = 7 mice per group. *p < 0.05.
Related to Figures S1 and S2 and Tables S1–S3.