Table 1. Exploring the reaction conditions a b .
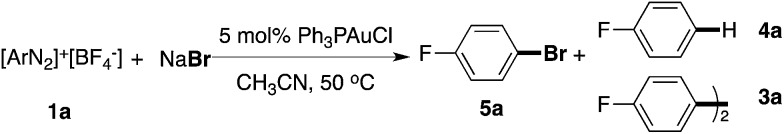
| ||||||
| Entry | Variations from above conditions | Time | Conv. (%) | 5a (%) | 3a (%) | 4a (%) |
| 1 | None | 5 h | 100 | 83 | 7 | <5 |
| 2 | Blue LED, No Ph3PAuCl | 12 h | 50 | <10 | Trace | 33 |
| 3 | LiBr instead of NaBr | 12 h | 100 | 78 | 8 | <5 |
| 4 | Acetone instead of ACN | 5 h | 100 | 11 | 37 | <5 |
| 5 | Ph3PAuNTf2 instead of Ph3PAuCl | 5 h | 100 | 68 | 10 | 7 |
| 6 | Ph3PAuNTf2 and 20 mol% bpy | 12 h | 100 | 63 | 8 | 15 |
| 7 | 3 mol% Ph3PAuCl | 5 h | 100 | 81 | 7 | <5 |
| 8 | 1 mol% Ph3PAuCl | 5 h | 100 | 63 | 13 | 9 |
| 9 | No light (darkness) | 5 h | 100 | 76 | 8 | <5 |
aReaction conditions: 1 (0.1 mmol), NaBr (0.4 mmol), cat. Au (5 mol%) in acetonitrile (ACN), 50 °C.
b 19F NMR yield with benzotrifluoride as the internal standard.
