Table 4. Photo-induced C–C bond formation of alkyl bromide.
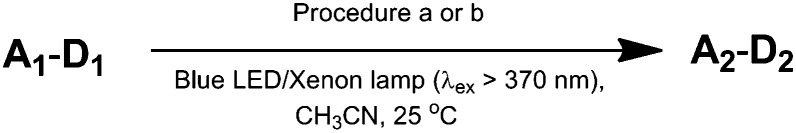
| |||||
| Entry | Cat. | Reaction time (h) | Substrate | Product | Conv.; yield f (%) |
| 1 a , c | Pd-N-1 | 10 |
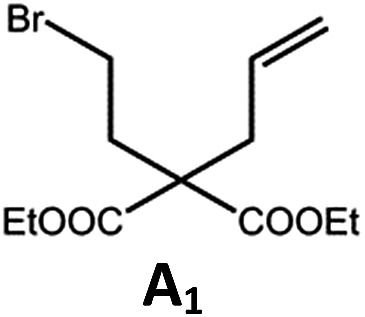
|
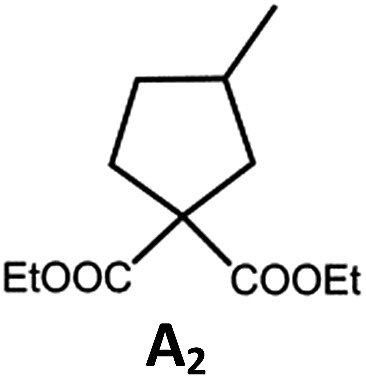
|
90; 83 |
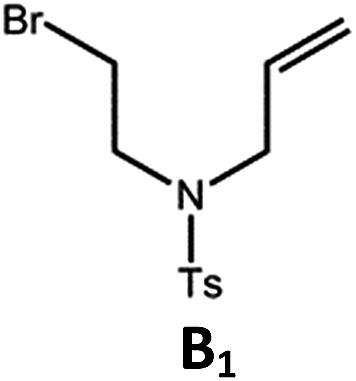
| 2 a , d | Pd-B-1 | 10 | 0; 0 | ||
| 3 d , e | Pd-B-1 | 10 | 30; 15 | ||
| 4 b , c | Pd-N-1 | 10 |
|
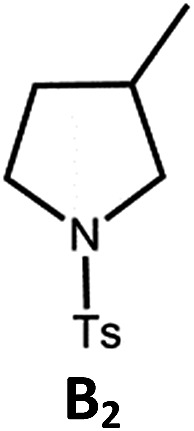
|
84; 63 |
| 5 b , c | Pd-N-1 | 10 |
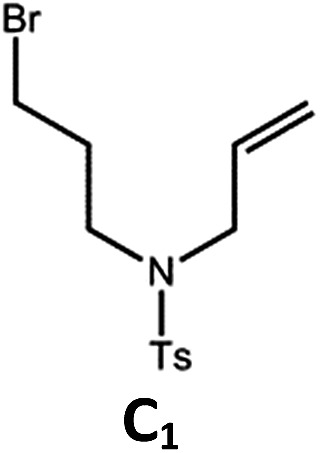
|
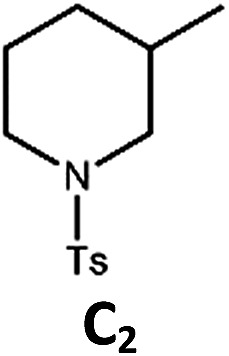
|
88; 66 |
| 6 a , c | Pd-N-1 | 20 |
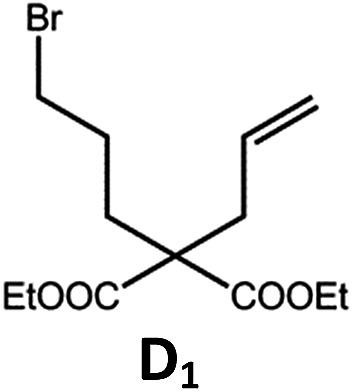
|
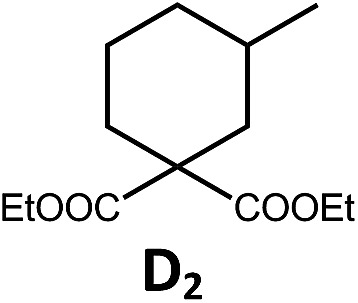
|
90; 68 |
aProcedure a: Pd(ii) complex (1 mol%) iPr2NEt (2 equiv.), CH3CN.
bProcedure b: Pd(ii) complex (1 mol%) iPr2NEt (5 equiv.), HCO2H (2 equiv.), CH3CN.
cThe reaction mixture was irradiated with a blue LED (λmax = 462 nm)
dThe reaction mixture was irradiated with a xenon lamp (λex > 370 nm).
eThe procedure is the same as a except TMEDA was used instead of iPr2NEt.
fDetermined by 1H NMR spectroscopy using 4,4′-dimethyl-2,2′-bipyridine as an internal standard. Ts = Tosylate.
