Table 5. Photo-catalysis of [2 + 2] styrene cycloaddition a .
| Entry | Cat. | Reaction time (h) | Substrate | Product | Conv.; yield (%) (d. r.) e |
| 1 | Pd-B-1 | 3 |
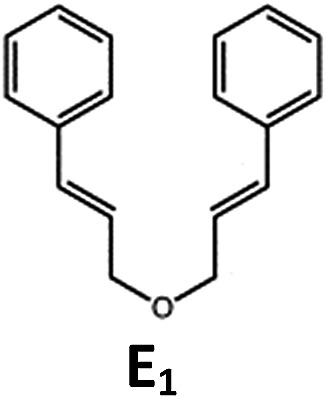
|
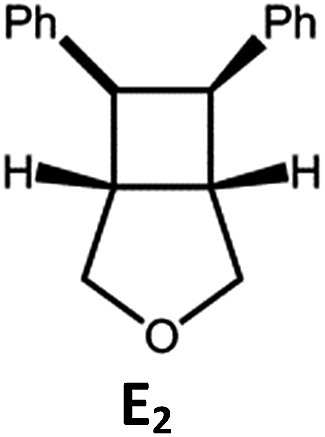
|
100; 88 (4 : 1) b |
| 4 | 27; 14 (4 : 1) d | ||||
| 2 | Pd-N-1 | 4 | 100; 97 (4 : 1) c | ||
| 4 | 79; 73 (4 : 1) d | ||||
| 3 | Pd-B-4 | 4 | 0; 0 b | ||
| 4 | 0; 0 c | ||||
| 4 | fac-Ir(ppy)3 | 4 | 100; 77 (4 : 1) c | ||
| 4 | 76; 62 (4 : 1) d | ||||
| 5 | — | 4 | 0; 0 b | ||
| 6 | Pd-B-1 | 8.5 |
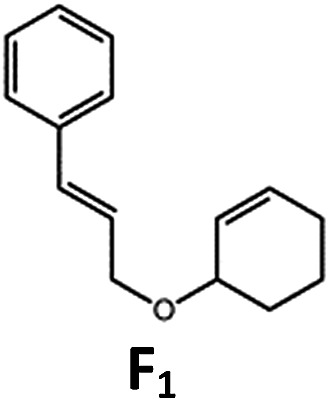
|
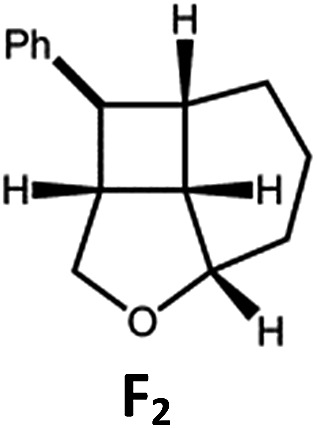
|
90; 74(>10 : 1) b |
| 7 | Pd-N-1 | 24 | 100; 82 (7 : 1) c | ||
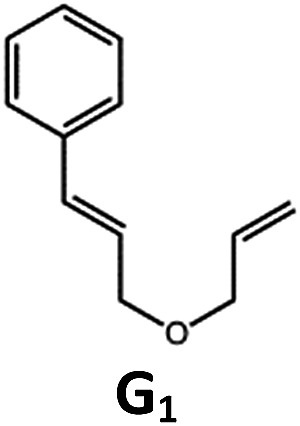
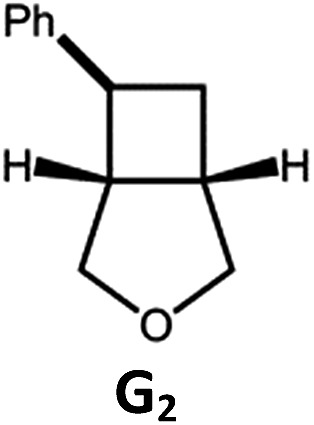
| 8 | Pd-B-1 | 8.5 |
|
|
100; 94 (6 : 1) b |
| 9 | Pd-N-1 | 24 | 100; 97 (5 : 1) c | ||
| 10 | fac-Ir(ppy)3 | 24 | 100; 61 (6 : 1) c | ||
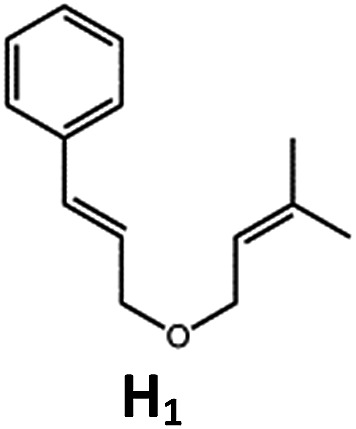
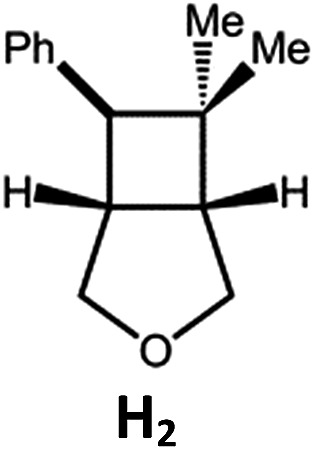
| 11 | Pd-B-1 | 8.5 |
|
|
100; 85 (7 : 1) b |
| 12 | Pd-N-1 | 16 | >99; 80 (7 : 1) c | ||
| 13 | fac-Ir(ppy)3 | 16 | >99; 79 (6 : 1) c |
aReaction condition: metal complex (1 mol%), substrate (0.01 mol dm–3) in CH3CN.
bXenon lamp (λex > 350 nm) as the light source.
cblue LED (λem = 462 nm) as the light source.
d23 W compact fluorescent lamp (CFL) as the light source.
eProduct yield determined by 1H NMR spectroscopy using 4,4′-dimethyl-2,2′-bipyridine as an internal standard.
