Table 2. Synthesis of cage B(4)-alkynylated o-carboranes using alkynyl bromide a .
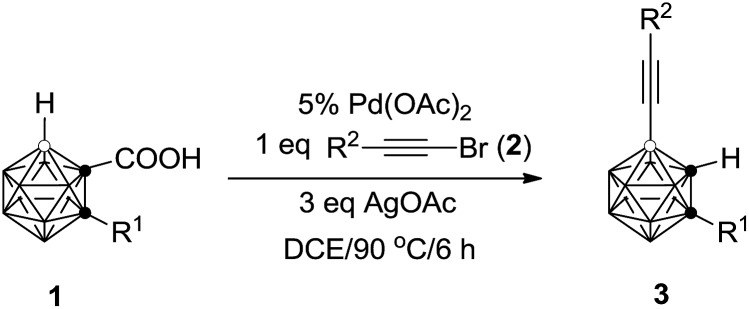
| |||
| Entry | R1 | R2 (2) | Isolated yield (%) |
| 1 | Me (1a) | iPr3Si | 81 (3a) |
| 2 | Et (1b) | iPr3Si | 76 (3b) |
| 3 | iPr (1c) | iPr3Si | 75 (3c) |
| 4 | Bn (1d) | iPr3Si | 73 (3d) |
| 5 | Ph (1e) | iPr3Si | 77 (3e) |
| 6 | 4-MeC6H4 (1f) | iPr3Si | 82 (3f) |
| 7 | 3,5-(CH3)2C6H3 (1g) | iPr3Si | 70 (3g) |
| 8 | 4-CF3C6H4 (1h) | iPr3Si | 81 (3h) |
| 9 | 4-ClC6H4 (1i) | iPr3Si | 72 (3i) |
| 10 | 4-MeOC6H4 (1j) | iPr3Si | 78 (3j) |
| 11 | 1-Naphenyl (1k) | iPr3Si | 40 (3k) |
| 12 | 2-Thiophenyl (1l) | iPr3Si | 54 (3l) |
| 13 | EtCH C(Et) (1m) | iPr3Si | 80 (3m) |
| 14 | H (1n) | iPr3Si | Messy |
| 15 | Me3Si (1o) | iPr3Si | 41 b (3n) |
| 16 | Me (1a) | t BuMe2Si | 70 (3p) |
| 17 | Me (1a) | Me3Si | N.R. c |
aReactions were conducted on a 0.2 mmol scale of 1 in a closed flask.
bMe3Si was removed after work up.
cN.R. = no reaction.
