Table 3. Optimization of reaction conditions using terminal alkynes a .
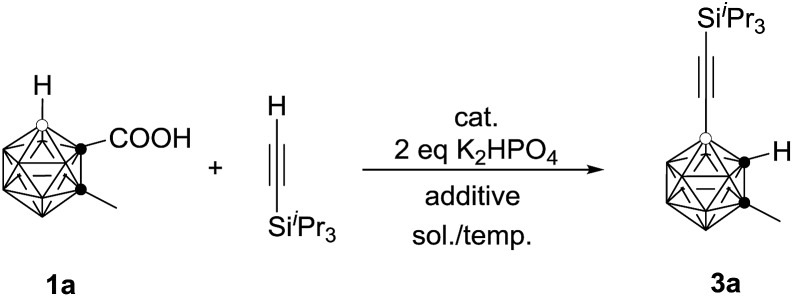
| |||||
| Entry | Cat (mol%) | Additive (equiv.) | Solvent | Temp (°C) | Yield b (%) |
| 1 c | Pd(OAc)2 (5) | AgOAc (3) | DCE | 90 | N.R. |
| 2 | Pd(OAc)2 (5) | AgOAc (3) | DCE | 90 | 30 |
| 3 | Pd(OAc)2 (5) | AgOAc (3) | DCE | 90 | 56 d |
| 4 | Pd(OAc)2 (5) | AgOAc (3) | DCE | 90 | 75 d , e |
| 5 | Pd(OAc)2 (5) | AgOAc (3) | Toluene | 90 | 78 d , e |
| 6 | Pd(OAc)2 (5) | AgOAc (3) | Toluene | 80 | 86 d , e |
| 7 | Pd(OAc)2 (5) | AgOAc (3) | Toluene | 70 | Trace |
| 8 | Pd(OAc)2 (3) | AgOAc (3) | DCE | 90 | 18 |
| 9 | Pd(TFA)2 (5) | AgOAc (3) | DCE | 90 | 26 |
| 10 | Pd2(dba)3 (5) | AgOAc (3) | DCE | 90 | 21 |
| 11 | Pd(OAc)2 (5) | Ag2CO3 (2) | DCE | 90 | 15 |
| 12 | Pd(OAc)2 (5) | Ag2O (2) | DCE | 90 | 12 |
| 13 | Pd(OAc)2 (5) | AgNO3 (3) | DCE | 90 | Trace |
aReactions were conducted on a 0.05 mmol scale of 1a in 0.5 mL of solvent in the presence of 2 equiv. of K2HPO4 in a closed flask for 10 h; DCE = 1,2-dichloroethane; TFA = trifluoroacetate; dba = dibenzylideneacetone.
bGC yields.
cWithout K2HPO4.
dTerminal alkyne was added dropwise by a syringe pump over a period of 10 h.
eTwo equiv. of terminal alkyne was added.
