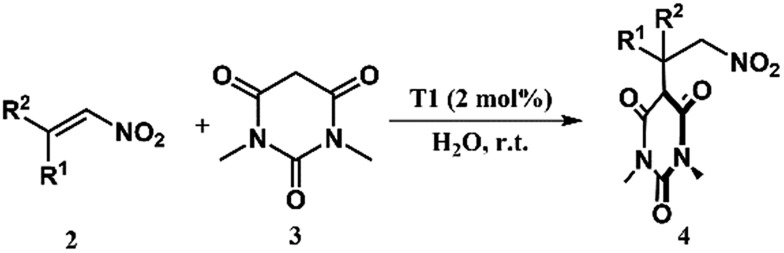
Table 1. Catalytic Michael addition reactions of 1,3-dimethylbabrbituric acid with different nitro-alkenes a .
| |||||||
| Entry | R1 | R2 | Product | Time (min) | Yield
b
(%) |
||
| Blank | With T2 | With T1 | |||||
| 1 | 1-Pyrenyl | H | 4a | 72 h | 14 | — | 20 |
| 2 | 1-Naphthyl | H | 4b | 90 | 15 | 22 | 41 |
| 3 | Ph | Me | 4c | 60 | 18 | 27 | 59 |
| 4 | 4-Me-Ph | H | 4d | 15 | 10 | — | 50 |
| 5 | 4-MeO-Ph | H | 4e | 30 | 13 | 20 | 54 |
| 6 | 2-Furanyl | H | 4f | 10 | 12 | — | 60 |
aNitro-olefins 2 (0.02 mmol), 1,3-dimethylbarbituric acid 3 (0.02 mmol), catalyst T1 (2 mol%), and water (1 mL), at r.t. with stirring.
bCrude yields were determined by 1H NMR studies.
