Abstract
Programmed cell death-ligands 1 (PD-L1) is a key immune checkpoint protein and a promising therapeutic target for malignancy tumor immunotherapy. The prognostic value of PD-L1 in patients with bone and soft tissue sarcoma remains controversial. Therefore, this meta-analysis is conducted to evaluate the associations of PD-L1 expression with overall survival (OS), progression-free survival (PFS), and clinicopathological characteristics of sarcoma
A comprehensive literature search of PubMed, Web of Science, Embase, and Cochrane Library was conducted for relevant studies. A total of 14 studies published from 2013 to 2017 were included. The pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were extracted from included studies to assess the association between PD-L1 expression and OS, PFS of patients with sarcoma. Other relevant data were extracted to evaluate the correlations of PD-L1 expression with risk and clinicopathological characteristics of sarcoma. Stata 12.0 software was applied to calculate the strength of association between PD-L1 expression and sarcoma.
In total, 14 articles containing 15 independent studies and 1,451 patients were included in this meta-analysis. We found that the high PD-L1 expression was associated with poorer overall survival (HR 1.27, 95% CI: 0.70–1.84 P = .000) and poorer events-free survival (HR 2.05, 95% CI: 1.55–2.70, P = .000) in bone and soft-tissue sarcoma patients. Additionally, we conducted subgroup analysis according to histology type, ethnicity, target of PD-L1 assessment, cutoff, the significant correlations with poor overall survival and events-free survival were also observed. In contrast none of the clinicopathological characteristics (gender, age, tumor site, tumor grade, tumor depth, tumor necrosis rate, metastasis, recurrence, chemotherapy, radiotherapy) was found to be associated with PD-L1 expression in our analysis.
The findings from this meta-analysis indicate that PD-L1 expression might be a useful predicative factor of poor prognosis for patients with bone and soft tissue sarcoma.
Keywords: bone and soft tissue sarcoma, meta-analysis, prognosis, programmed cell death ligand 1 (PD-L1)
1. Introduction
Sarcomas are a group of malignant tumors of mesenchymal origin and characterized by heterogeneous subtypes with various cytogenetic profiles. Skubitz and D’Adamo divided sarcomas into 2 general categories: primary bone sarcoma and soft-tissue-sarcoma in 2007.[1] In recent years, with the development of surgical techniques and the emergence of effective chemotherapy, combining chemotherapy, radiotherapy with surgery became the standard treatment for sarcoma. However, the overall survival still was stagnant and the 5-years probability of local recurrence, metastasis remained high.[2,3] Meanwhile, the prognosis is poorer in the majority of these cases, the average survival period after developing recurrence and metastasis is less than 1 year.[4] The limitations in current treatment of sarcoma desperately need breakthroughs, particularly for case with metastatic and relapse disease, to treat these devastating diseases.
Immunotherapy is regarded as an emerging therapeutic option in oncology and immune escape is the important biological process for malignant tumor progression.[5,6] Recent studies have proved programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1) play a pivotal role in the immune surveillance by inhibiting the function of T cell, resulting in immune system impairment.[7,8] Since anti-PD-1 antibodies have been approved in non-small-cell lung cancer, melanoma with efficacy and safety profiles, and the PD-L1 expression significantly correlate with poor survival in several solid tumors, blockade the PD-1/PD-L1 pathway shows vast potential for tumor immunotherapy.[9–12] There is no doubt that an effective prognostic factor is important for clinicians to make the individual treatment strategy, and the correlation between PD-L1 expression and survival of sarcoma patients has also been investigated, but the prognostic value of PD-L1 expression remains a controversial subject. Because of the discrepancy in PD-L1 assessment assay and relatively small sample sizes of each individual study, we performed a meta-analysis including 14 studies with 1451 patients to evaluate the association between PD-L1 expression and survival prognosis or clinicopathological parameters of sarcoma patients.
2. Results
2.1. Search result and characteristics of studies
In this meta-analysis, we identified a total of 511 potentially relevant articles after the initial search of PubMed, Embase, Cochrane library, and Web of Science. After screening the titles and abstracts, 472 studies were excluded because of the duplicate or irrelevant. Thirty-nine potentially eligible articles were further reviewed in detail, 25 articles were excluded, including 14 articles without sufficient data, 2 recruiting overlapping patients, 4 articles including patients with other tumors, 5 articles that were not relevant. As a result, 14 articles containing 15 independent studies and 1451 patients were selected for this meta-analysis. The detailed diagram of filtering process is presented in Fig. 1.
Figure 1.
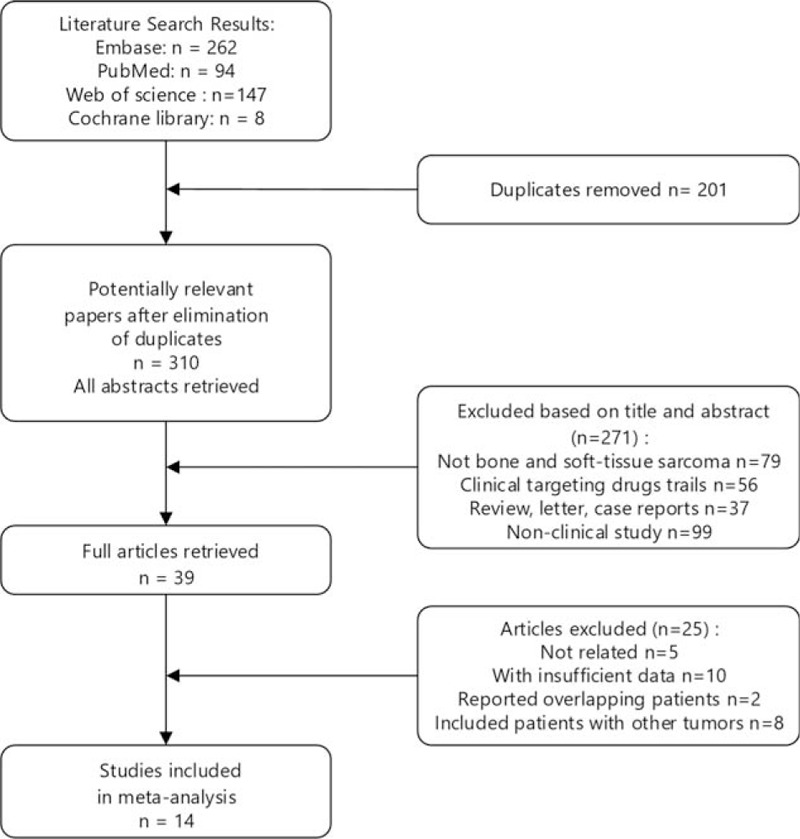
Follow diagram for selection of studies.
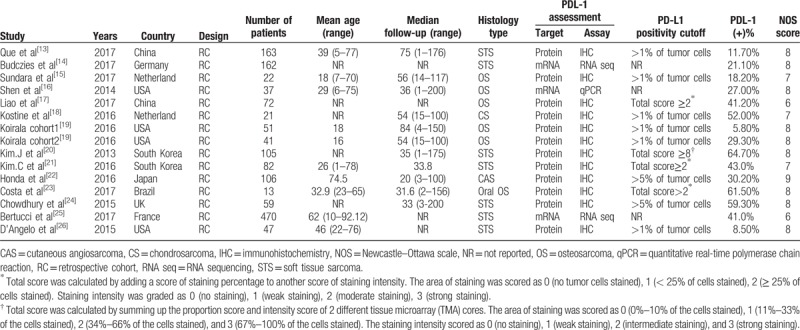
The basic characteristics of the eligible studies are summarized in Table 1.[13–26] The sample sizes of each study ranged from 13 to 470 patients. And a total of 1451 patients from Asian, Europe, and America were enrolled in the studies. Because the study was carried out by Koirala, 2 cohorts of patients were enrolled, and PD-L1 was reported independently, so they were analyzed as 2 individual studies. All 15 of the eligible studies for the analysis were retrospectively designed. The proportion of PD-L1 positivity in the above studies ranged from 5.87% to 64.7%. The histological category of tumors included osteosarcoma (6 studies), soft tissue sarcoma (8 studies), and chondrosarcoma (1 study). Five studies were reported on Asians, and 10 studies on Caucasians. The PD-L1 protein expression was detected by immunohistochemistry (IHC) in 12 studies, while the remaining study used quantitative real-time PCR (1 study) and RNA sequencing (2 studies) to survey the PD-L1 mRNA expression. The survival endpoints were reported by different effect size including overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS), recurrence-free survival(RFS), and events-free survival (EFS). The HR estimations for 6 studies were directly reported, whereas the other studies provided survival curves and P value with which to calculate HR with 95% CI.
Table 1.
Characteristics of the studies included in the meta-analysis.
2.2. Association between PD-L1 expression and overall survival
We investigated the correlation between PD-L1 expressions and OS in sarcoma patients. A total of 10 studies with 911 sarcoma patients were included in the analysis of overall survival. The combined HR of enrolled studies showed that PD-L1 expression was associated with poor OS (HR 1.35, 95% CI: 0.98–1.72, P = .000). Furthermore, a significant heterogeneity was detected among the studies with using random effects model for the analysis (I2 = 48.0%, P = .044); therefore, we performed the subgroup analysis to clarify the potential sources (Fig. 2A).
Figure 2.

Meta-analysis of the effect of PD-L1 expression on overall survival. The forest plot of hazard ratio for overall survival of patient with sarcoma (A), subgroup analysis stratified by the histological subtype (B), subgroup analysis stratified by the histological subtype (C). Subgroup analysis stratified by the cutoff (D). Subgroup analysis stratified by the target of PD-L1 assessment (E). PD-L1 = programmed cell death-ligands 1.
2.3. Subgroup analysis
The subgroup analysis stratified by the histological subtype indicates that the PD-L1 was a negative prognostic factor for soft tissue sarcoma and osteosarcoma, the pooled HR estimate for overall survival in soft tissue sarcoma group was 1.41 (95% CI: 0.92–1.90, P = .000) with no significant heterogeneity (I2 = 42.8%, P = .120), and the pooled HR estimate for overall survival in osteosarcoma group was 1.93 (95% CI: 1.18–2.68, P = .000) with no heterogeneity (I2 = 0.0%, P = .486). Meanwhile, no significant correlation between PD-L1 and osteosarcoma was found in the Chondrosarcoma subgroup analyses (HR = 0.36, 95% CI: 0.53–1.25, P = .426), as shown in Fig. 2B. The subgroup analysis stratified by the ethnicity indicated that the PD-L1 expression was associated with poor overall survival in either Asian or Caucasian patients. The pooled HR estimate for Asian patients group was 1.51 (95% CI: 1.03–1.99, P = .000) with high heterogeneity (I2 = 61.2%, P = .035), and for Caucasian patients group was 1.10 (95% CI: 0.50–1.69, P = .000) with low heterogeneity (I2 = 31.5%, P = .211), as shown in Fig. 2C. In the subgroup analysis stratified by cutoff, the pooled HR estimate for cutoff <5% of tumor cells group was 0.85 (95% CI: 0.13–1.57, P = .021) with medium heterogeneity (I2 = 44.5%, P = .1443), and for studies without clear cutoff the heterogeneity was negligible (I2 = 0.0%, P = .603). Meanwhile, the pooled HR estimate for cutoff ≥5% of tumor cells group was 1.44 (95% CI: 0.95–1.93, P = .000) with medium heterogeneity (I2 = 64.1%, P = .039), as shown in Fig. 2D. To clarify the impact of a different PD-L1 assessment on the results, we conducted subgroup analysis stratified by the target of assessment. The pooled HR was 1.25 (95% CI: 0.85–1.66, P = .000) for Protein, with high heterogeneity (I2 = 55.0%, P = .030), and 1.90 (95% CI: 0.94–2.85, P = .000) for mRNA, with negligible heterogeneity (I2 = 0.0%, P = .603) (Fig. 2E).
2.4. Association between PD-L1 expression and event-free survival
Although 7 studies evaluated the association the PD-L1 expression with different effect size, including the events-free survival, disease-free survival, metastasis-free survival, and recurrence-free survival, the event was defined as local recurrence, distant metastasis, or death, which is accord with the DFS, MFS, RFS. Therefore, we combined the HRs as the effect size to evaluate survival outcome. The heterogeneity analysis showed low heterogeneity among the studies (I2 = 30.1%, P = .198), and the fixed-effect model was applied for HR. The pooled HR estimate was 1.70 (95% CI: 1.24–2.150, P = .000) for the bone and soft-tissue sarcoma patients (Fig. 3). Overall, the meta-analysis showed a significant difference between positive and negative PD-L1 expression, revealed PD-L1 was a poor prognostic factor for events-free survival in sarcoma patients.
Figure 3.
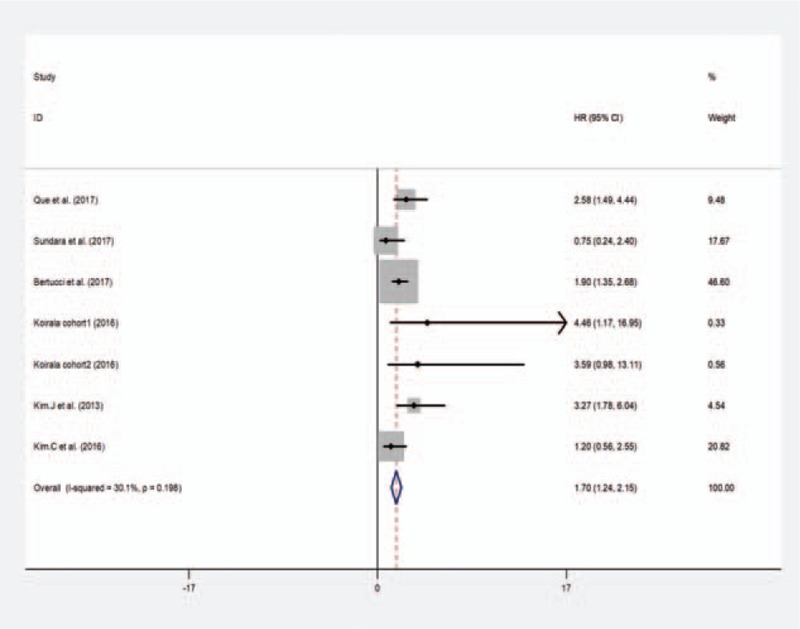
Meta-analysis of the effect of PD-L1 expression on events-free diseases survival.
2.5. Association between PD-L1 expression and clinicopathological parameters
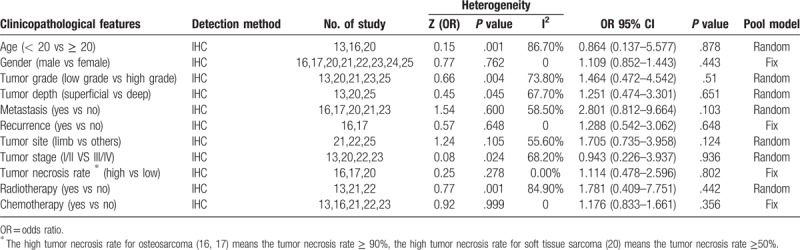
Seven studies assessed the association between PD-L1 expression and clinicopathological parameters. The clinical parameters analyzed included age, gender, tumor grade, tumor depth, tumor stage, tumor necrosis rate, tumor site, chemotherapy, radiotherapy, metastasis, recurrence. The pooled results of clinicopathological parameters are shown in Table 2. Furthermore, none of the clinicopathological parameters was found to be significantly correlated with PD-L1 expression. For the high heterogeneity group, a random effect model was used; meanwhile we also performed subgroup analysis, stratified by the histological subtype. The result still showed PD-L1 expression was not correlated with the clinicopathological parameters.
Table 2.
Relationship between PD-L1 protein expression and the clinicopathological feature.
2.6. Publication bias and sensitivity analysis
By using Egger tests, there is no publication bias affecting the hazard ratios for overall survival and events-free survival was present in the eligible studies. The P values for these tests were .679 and .163, respectively. At the same time, no publication biases were observed based on the visual evaluation of the Begg funnel plot (Fig. 4A, B).
Figure 4.
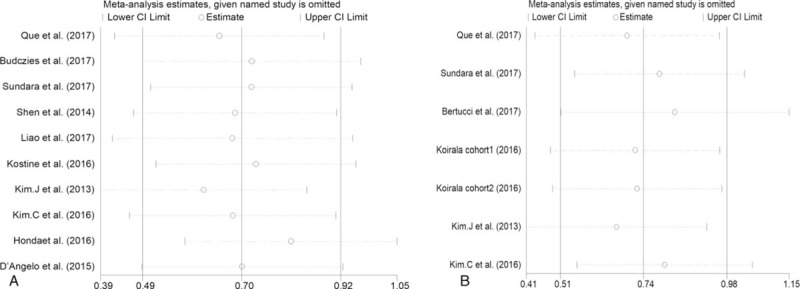
Sensitivity analysis of the correlation between PD-L1 expression and overall survival (A), events-free diseases survival (B).
Sensitivity analysis, by omitting 1 study at a time, was performed to detect the stability of the results. The results shown in Fig. 5A, B demonstrated that no unique study significantly affected the pooled HRs of OS and EFS, indicating that the results of the present meta-analysis are stable and credible.
Figure 5.
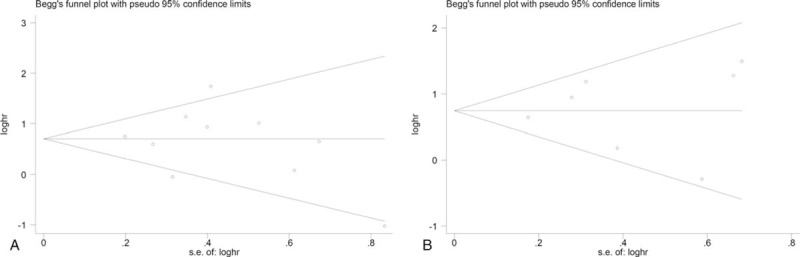
Begg funnel plot for publication bias of the association between PD-L1 expressions and overall survival (A) and events-free survival (B).
3. Discussion
Bone and soft tissue sarcoma are a rare heterogeneous neoplasms of mesenchymal derived, accounting for approximately 1% of all human malignance.[27] Although the overall survival rate has been raised with the development of effective chemotherapy and surgical techniques, but the cures for sarcoma patients by using the traditional strategy were stagnant for decades.[28–30] However the local recurrence and metastasis are still common and the average survival of these patients is poorer.[31] In the contrast, the immunotherapy has achieved tremendous success in solid tumors and been considered the foremost method in individualized medicine.[5,32] Understanding how immunology mechanism of action relates to sarcomas may reveal the potential for immunotherapy and to develop new effect treatment method for sarcoma.
PD-1 and its ligands, PD-L1 and PD-L2 overexpressed within the tumor microenvironment, can inhibit T-cell activation and proliferation and negatively regulate the immune response through various pathways in antitumor immunity, thereby losing its killing effect on tumor cells and protecting tumor cells from the host immunologic surveillance system.[33] Recently, the association between PD-L1 expressions and various solid tumors has been studied by numerous researches and the results have shown that expression of PD-L1 significantly correlates with poor prognosis.[9,34–36] However, its expression and impact on the survival outcome of patients with bone and soft tissue sarcoma remains inconsistent and conflicting. Multiple studies have shown that positive PD-L1 was associated with significantly poor prognosis,[13,14,17,20,21,26] but other studies could not confirm this finding. To achieve a reasonable conclusion, we conducted this meta-analysis and aimed to assess the correlation between PD-L1 expression and the prognosis of bone and soft tissue sarcoma patients.
According to the result of literature retrieval, our analysis combined14 studies (including 15 cohorts) with 1451 patients. The results reveal that PD-L1 expression is a negative prognostic factor in bone and soft tissue sarcoma with statistical significances for OS (HR = 1.35, 95% CI: 0.98–1.72), and EFS (HR = 1.70, 95% CI: 1.24–2.150). Due to the high heterogeneity among the overall survival of sarcoma patients, we preformed subgroup analysis according to different histological subtypes, ethnicities, target of PD-L1 assessment, and cut-off. Interestingly, for tumor histological subtype, the analysis results have shown a significant association between PD-L1 expression and the poor overall survival of patient with osteosarcoma, soft tissue sarcoma, with no significant heterogeneity (I2 = 0.0%, P = .486; I2 = 42.8%, P = .120), respectively. Therefore, we consider that the different histological subtype might be a factor that explains the heterogeneity. In the enrolled studies, the different cutoff and target of PD-L1 assessment were used to detect the expression of PD-L1, while the pooled HR for protein and cutoff ≥5%, <5% group show high heterogeneity (I2 = 55.0%, P = .030 and medium heterogeneity (I2 = 64.1%, P = .038; I2 = 44.5%, P = .144), respectively. We presume that even IHC is the common method used by including studies, PD-L1 positivity was assessed by using different antibodies, the sensitivities of the antibodies and the experimental procedures, different cutoff might be the factor which contributes to the heterogeneity.
According to the result of included studies in the meta-analysis, the result was consistent with multiple studies, however, expression of PD-L1 was a favorable prognostic factor and improved the survival in 1 study.[22] These inconsistent and conflicting results have several possible explanations: different histology type of tumor was selected to be investigated and different sample size from different areas of study which suggested that result of study might be debatable. Meanwhile, comparisons of different studies reporting PD-L1 expression are unconformity by the use of different methodologies, different thresholds, different antibodies. Additionally, tumor cells express PD-L1 by antitumor immune response and oncogene-driven mechanisms.[37,38] The former is induced by cytokines and dependent on the presence of T lymphocytes infiltration.[39] The latter does not depend on the presence of T lymphocytes infiltration and multiple mechanisms can lead to PD-L1 expression, we hypothesize that these inconsistent results might be partially due to the impact of PD-L1 expression on the different populations of T lymphocytes infiltration or active immune responses and different PD-L1 regulation at both transcription and translational levels. Further investigations will be required to clarify the underlying mechanism.
In published data, nearly all the clinicopathological features, including age, gender, tumor grade, tumor depth, tumor stage, tumor necrosis rate, tumor site, chemotherapy, radiotherapy, metastasis, recurrence, have been demonstrated associated with PD-L1 expression in sarcoma. However most of them were not strongly confirmed by other studies. Only 1 study [13] showed PD-L1 expression was associated with age. Meanwhile, PD-L1 expression was found to be associated with tumor necrosis rate also by only 1 study.[17] With regards to tumor grade, tumor stage, and tumor depth, significant results were both reported in 2 studies.[13,20] Besides, PD-L1 expression was found to be correlated with tumor metastasis in 5 studies.[15,17,19,20,23] However, when we combined the data together, none of the clinicopathological features mentioned above was associated with PD-L1 expression. The discordances among previous and current analysis may result from inadequate sample size, heterogeneous study population, and variable definitions of PD-L1 expression and different methodologies.
Thus, studies with larger sample size and standardized quantitative assays were still needed to assess the correlation between PD-L1 expression and clinicopathological parameters.
Although we made an effort to perform a comprehensive analysis, but there were some limitations to our analysis. First, significant heterogeneity was observed among studies, which will influence the conclusions of this study. To minimize the effect of the heterogeneity, a random effect model and subgroup analysis were applied for high heterogeneity group. Second, within each study, different methods of PD-L1 measurement were used to detect the expression of PD-L1. Despite that the common method was IHC, PD-L1 positivity was assessed using different antibodies, as the sensitivities of the antibodies, the experimental procedures, and cutoff are varied. Meanwhile, the immunohistochemical reagents, the scoring method, the cut-off values used to determine PD-L1 positivity also varied among the studies included in the analysis. Thus, these variances of different methodology may result in heterogeneity among the including studies of this meta-analysis and may lead to a potential bias. Third, the sample sizes of the studies eligible in the analysis were relatively small. However, the results of the sensitivity analysis results still remain stable and creditable after the sequential exclusion of each study. Fourth, not all of the HR with 95% CI was directly extracted from the studies, so if this was impracticable we had to extrapolate the HR via Kaplan–Meier curves or P values. The estimation might be less reliable than those reported directly. Finally, publication bias should be concerned, our meta-analysis was limited to articles published in English. Additionally, certain studies with negative results or some useless results may not be published in journals, which may result in publication bias. To minimize the effect, we conduct comprehensive literature searches as completely as possible by using PubMed, Embase, Web of Science, and the Cochrane Library. But no statistical significant publication bias was found in this meta-analysis. Nevertheless, it should be noted that we excluded the case reports, reviews, letter, and conference abstracts as it did not contain sufficient data for aggregation. In general, these above limitations may bring potential source of publication bias to the current meta-analysis. Therefore, the conclusion and results of the current meta-analysis should be interpreted with caution and need to be validated by more well-designed prospective studies with appropriate multivariate analyses.
4. Conclusion
In conclusion, this meta-analysis demonstrates that PD-L1 expression is significantly correlated with poor OS and EFS for patients with sarcoma, and PD-L1 expression may be a useful predicative factor of prognosis for bone and soft tissue sarcoma. This information may be beneficial to clinicians to choose individual treatment methods by anti-PD-1/PD-L1 therapy. Future adequately designed clinical studies with uniform assessment assay are needed to verify the role of PD-L1 expression in sarcoma.
5. Materials and methods
5.1. Literature search strategy
A comprehensive literature search was performed in the PubMed, Embase, Web of Science, and Cochrane databases with no language restrictions. Articles published before August 2017 were included in the meta-analysis. The following keywords were used as both text words and Medical Search Headings (Mesh terms) and were combined by using Boolean operators for the relevant literature: (“Sarcoma”[Mesh] OR “Sarcoma∗” OR “Soft tissue sarcoma∗” OR “Epithelioid Sarcoma∗” OR “Spindle Cell Sarcoma∗” OR “Osteosarcoma∗” OR “Osteosarcoma Tumor∗” OR “Osteogenic Sarcoma∗” OR “Chondrosarcoma∗” OR “Ewing sarcoma∗” OR “Ewing's Tumor∗” OR” Carcinosarcoma∗”OR “Leiomyosarcoma∗” OR “Angiosarcoma∗” OR “Desmoplastic Small Round-Cell Tumor∗” OR “Hemangiosarcoma∗” OR ”Lymphangiosarcoma∗” OR ” Malignant Lymphangioendothelioma∗” OR ” Myosarcoma∗” OR “Fibrosarcoma∗” OR “Synovial sarcoma∗” OR Malignant Fibrohistiocytic Tumor∗” OR ” Malignant Fibrous Histiocytomas∗” “Liposarcoma∗” OR ” Dedifferentiated Liposarcoma∗” OR ” Pleomorphic Liposarcoma”) AND (“PD-L1” OR “B7-H1” OR “CD274” OR “programmed cell death 1 ligand 1 protein” OR “CD274 Antigen∗” OR “B7H1 Antigen∗” OR “PD-L1 costimulatory protein” OR “B7-H1 Immune Costimulatory Protein” OR “PD-L1 Costimulatory Protein”).
5.2. Eligibility criteria and quality assessment
The eligible studies for the analysis had to be in accordance with the following criteria: studies whose entire populations comprised patients with histologically confirmed bone and soft tissue sarcoma; studies providing sufficient data regarding the correlation between PD-L1 and clinicopathological features and overall survival (OS); studies that revealed the prognosis provided the sufficient information to estimate hazard ratio (HR) about survival outcome. The language of literature was restricted to English. Studies were excluded from the analysis if they meet the following: reported overlapping patients; nonclinical research; reviews, case reports, letters and articles from conferences; with insufficient information. When the same patient source was included in different publications, the most recent and largest study was included in analysis. Two independent reviewers (CZ and WY) verified the included studies met the above criteria for subsequent analysis.
Since all the studies included were cohort studies, the quality of each study was evaluated using Newcastle–Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Based on the assessment of each study in selection, comparability, and exposure, a score up to 9 points was appointed. The quality assessment was carried out independently by 2 reviewers (CZ and WY). The disagreement was resolved by consensus (CZ, WY, and SZ).
5.3. Data extraction
All relevant data of the eligible articles were extracted by 2 independent researchers (CZ and WY), and any disagreements were resolved by consultation. The following data was extracted: basic information of each article including first author, year of publication, country, number of patients, follow-up duration, and study design; data of patient and tumor including age, gender, tumor grade, tumor depth, tumor stage, tumor necrosis rate, tumor site, chemotherapy, radiotherapy, metastasis, recurrence; prognosis outcome measures including overall survival, disease-free survival, events-free survival, recurrence-free survival, metastasis-free survival, Kaplan–Meier curves and P values; other data including the assay methods and PD-L1 positivity expression cut-off value.
5.4. Statistical analyses
To evaluate the prognostic significance of PD-L1 expression, HRs and theirs 95% CIs were used to determine the association between PD-L1 expression and survival, and pooled ORs and its 95% CIs were used to assess the correlation between PD-L1 and clinicopathological parameters of sarcoma patients. When the HRs were given explicitly in the article, we used the original data, or calculated the HRs with 95% CIs from available survival data in the articles or from Kaplan–Meier curves by using the methods reported by Tierney et al.[40]
Statistical heterogeneity among each study was evaluated by using Cochran Q test and Higgins I2 statistic. If P >.1 and I2 < 50%, it indicated a significant heterogeneity between studies and a random effects model was selected to combine the data in these cases. Otherwise, the heterogeneity was not significant and a fixed effects model was used. Meanwhile, the subgroup analysis was further performed to explore the heterogeneity source. Publication bias was assessed by Egger test and Begg test. All statistical analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX). P values <.05 were considered statistically significant.
Acknowledgments
The authors thank all the authors whose publications were included in our meta-analysis.
Author contributions
Conceptualization: ChuanXi Zheng, Wei You, peng wan, Yuchen Zheng, Wei Li.
Data curation: ChuanXi Zheng, Wei You, peng wan, Xiaochun Jiang, Jinquan Chen, Yuchen Zheng, Wei Li, Jifeng Tan.
Formal analysis: ChuanXi Zheng, Wei You, Jifeng Tan.
Funding acquisition: ChuanXi Zheng, Wei You.
Investigation: ChuanXi Zheng, Wei You.
Methodology: ChuanXi Zheng, Wei You.
Project administration: ChuanXi Zheng, Wei You.
Resources: ChuanXi Zheng, Wei You.
Software: ChuanXi Zheng.
Supervision: ChuanXi Zheng, Shiquan Zhang.
Validation: ChuanXi Zheng, Wei You, Shiquan Zhang.
Visualization: ChuanXi Zheng, Wei You, Shiquan Zhang.
Writing – original draft: ChuanXi Zheng, Wei You.
Writing – review & editing: ChuanXi Zheng, Wei You, Shiquan Zhang.
Footnotes
Abbreviations: CAS = cutaneous angiosarcoma, CI = confidence interval, CS = chondrosarcoma, DFS = disease-free survival, EFS = event-free survival, HR = hazard ratio, MFS = metastasis-free survival, OR = odds ratio, OS = osteosarcoma, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival, STS = soft tissue sarcoma.
CZ and WY contributed equally to this study.
This study was mainly supported by grant support from the Shenzhen Health and Family Planning Commission Project (201501014).
Ethnical statement: This study did not need consent of ethics committee or institutional review board, because the research was a meta-analysis which belonged to a review article.
The authors have no conflicts of interest to disclose.
References
- [1].Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc 2007;82:1409–32. [DOI] [PubMed] [Google Scholar]
- [2].Daigeler A, Zmarsly I, Hirsch T, et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer 2014;110:1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ghosn M, El RE, Kourie HR. Immunotherapies in sarcoma: updates and future perspectives. World J Clin Oncol 2017;8:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burgess M, Gorantla V, Weiss K, et al. Immunotherapy in sarcoma: future horizons. Curr Oncol Rep 2015;17:1–0. [DOI] [PubMed] [Google Scholar]
- [5].Hodi FS, O’Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015;373:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hatam LJ, Devoti JA, Rosenthal DW, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T-cells and PD-1/PD-L1/L2 expression. Clin Cancer Res 2012;18:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang W, Peng C, Wan Y, et al. Longitudinal fluctuations in PD1 and PD-L1 expression in association with changes in anti-viral immune response in chronic hepatitis B. BMC Gastroenterol 2012;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang Y, Zhang SD, Mccrudden C, et al. The prognostic significance of PD-L1 in bladder cancer. Oncol Rep 2015;33:3075–84. [DOI] [PubMed] [Google Scholar]
- [10].Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Oncotargets Ther 2014;2014:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751–5. [DOI] [PubMed] [Google Scholar]
- [13].Que Y, Xiao W, Guan YX, et al. PD-L1 expression is associated with FOXP3+ regulatory T-cell infiltration of soft tissue sarcoma and poor patient prognosis. J Cancer 2017;8:2018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Budczies J, Mechtersheimer G, Denkert C, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology 2017;6:e1279777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sundara YT, Kostine M, Cleven AH, et al. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol Immunother 2017;66:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shen JK, Cote GM, Choy E, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2014;2:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liao Y, Chen L, Feng Y, et al. Targeting programmed cell death ligand 1 by CRISPR/Cas9 in osteosarcoma cells. Oncotarget 2017;8:30276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kostine M, Cleven AH, de Miranda NF, et al. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod Pathol 2016;29:1028–37. [DOI] [PubMed] [Google Scholar]
- [19].Koirala P, Roth ME, Gill J, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep 2016;6:30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim JR, Moon YJ, Kwon KS, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 2013;8:e82870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim C, Kim EK, Jung H, et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016;16:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Honda Y, Otsuka A, Ono S, et al. Infiltration of PD-1-positive cells in combination with tumor site PD-L1 expression is a positive prognostic factor in cutaneous angiosarcoma. Oncoimmunology 2017;6:e1253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Costa Arantes DA, Gonçalves AS, Jham BC, et al. Evaluation of HLA-G, HLA-E, and PD-L1 proteins in oral osteosarcomas. Oral Surgery Oral Medicine Oral Pathology & Oral Radiology 2016;123:e188. [DOI] [PubMed] [Google Scholar]
- [24].Chowdhury F, Dunn S, Mitchell S, et al. PD-L1 and CD8+PD1+lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. OncoImmunology 2015;4:e1029701. [Google Scholar]
- [25].Bertucci F, Finetti P, Perrot D, et al. PDL1 expression is a poor-prognosis factor in soft-tissue sarcomas. Oncoimmunology 2017;6:e1278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].D’Angelo SP, Shoushtari AN, Agaram NP, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol 2015;46:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bannasch H, Eisenhardt SU, Grosu AL, et al. The diagnosis and treatment of soft tissue sarcomas of the limbs. Deutsches Rzteblatt Int 2011;108:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Torre L, Bray F, Siegel R, et al. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [29].Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112–28. [DOI] [PubMed] [Google Scholar]
- [30].O’Day K, Gorlick R. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther 2009;9:511–23. [DOI] [PubMed] [Google Scholar]
- [31].Tsukushi S, Nishida Y, Urakawa H, et al. Prognostic significance of histological invasion in high grade soft tissue sarcomas. Springerplus 2014;3:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Flemming A. Cancer: PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov 2012;11:601. [DOI] [PubMed] [Google Scholar]
- [34].Zhang L, Qiu M, Jin Y, et al. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int J Clin Exp Pathol 2015;8:11084–91. [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou C, Tang J, Sun H, et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget 2017;8:58457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thoma C. Prostate cancer: PD-L1 expression is common and indicates poor prognosis. Nat Rev Urol 2016;13:5. [DOI] [PubMed] [Google Scholar]
- [37].Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 2016;47:78. [DOI] [PubMed] [Google Scholar]
- [38].Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lastwika KJ, Rd WW, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT/mTOR pathway in non-small cell lung cancer. Cancer Res 2016;76:227–38. [DOI] [PubMed] [Google Scholar]
- [40].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]


