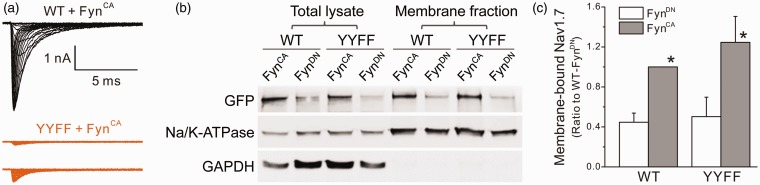
Figure 5.
Substitution of both tyrosine residues (Nav1.7rG-YYFF) dramatically reduced sodium currents recorded in HEK 293 cells, despite similar surface expressions between WT and mutant channels. (a) The representative sodium currents recorded from HEK 293 cells transfected by Nav1.7rG WT or YYFF plus FynCA. Cells expressing Nav1.7rG-WT channels generated decent sodium currents (representative raw traces in black), while sodium currents recorded from HEK 293 cells expressing Nav1.7rG-YYFF were extremely small (representative raw sodium currents of YYFF mutant recorded from two transfected HEK293 cells were shown in purple). (b) The representative Western blot image of total expression and cell surface expression of WT and YYFF mutant channels transiently transfected in HEK 293 cells with FynCA or FynDN. FynCA elevated the total and surface expressions of both WT and mutant channels, and the cell surface expression of YYFF mutant was not different to that of WT channels. (c) The histogram of membrane-bound WT and YYFF mutant channels expressed in HEK 293 cells. The surface expression level of WT channels cotransfected with FynCA was set as 1; and the membrane expression of WT/FynDN, YYFF/FynDN, and YYFF/FynCA was 0.45 ±0.09, 0.50 ± 0.19, and 1.25 ± 0.26, respectively. Data are obtained from four experiments and presented as mean ± SE, *p < 0.05 WT/FynCA or YYFF/FynCA versus corresponding channels cotransfected with FynDN by one-way ANOVA test. WT: wild type; YYFF: Nav1.7rG-Y1470F/Y1471F; GFP: Green Fluorescent Protein; GAPDH: glyceraldehyde phosphate dehydrogenase.