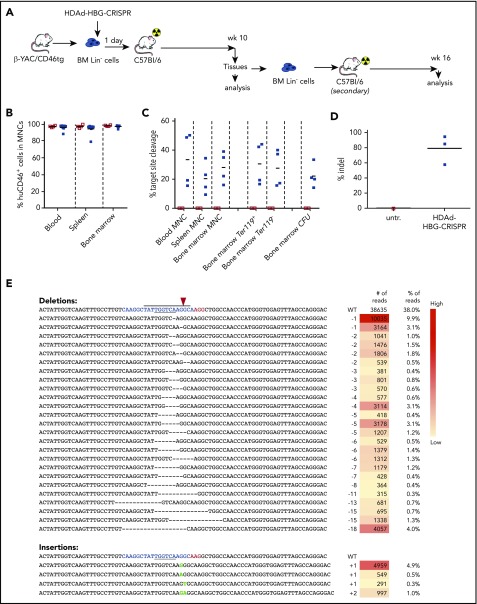
Figure 2.
Ex vivo transduction of β-YAC/CD46 Lin−cells with HDAd-HBG-CRISPR and subsequent transplantation. (A) Schematic diagram of the experiment. Bone marrow was harvested from β-YAC/CD46 mice, and Lin− cells were isolated by magnetic-activated cell sorting. Lin− cells were transduced with HDAd-HBG-CRISPR vector at a multiplicity of infection of 500 vp’s per cell (blue squares) or were left untransduced (empty red squares). After 1 day in culture, 1 × 106 transduced cells per mouse were transplanted into lethally irradiated C57BL/6 mice. Animals were euthanized at week 10, and bone marrow Lin− cells were transplanted into secondary recipients that were subsequently followed for 16 weeks. (B) Engraftment at week 10 based on the percentage of human CD46+ cells in mononuclear cells of blood, spleen, and bone marrow. (C) Percentage of HBG target site cleavage measured by T7E1 assay at week 10 after transplantation in the indicated samples. Each symbol represents an individual mouse. Cells from colonies were pooled, and genomic DNA was isolated. (The corresponding polyacrylamide gels for the graphs are shown in supplemental Figure 4.) (D) Percentage of total HBG indels obtained by deep sequencing of DNA from total bone marrow mononuclear cells at week 10 after transplantation. Each symbol is an individual animal. (E) Top 30 most frequent indels found in mouse #976 (indel percentage = 58%). The blue sequence shows the target of the sgRNA with the TGACCA BCL11A binding motif underlined. The horizontal bold black line indicates the −114 to −102 HPFH. The CRISPR/Cas9 cleavage site is marked by a red arrowhead. The right panels show the number and percentage of reads for the corresponding indel. A complete list of the indels in all 3 mice is provided in supplemental Table 3. CFU, colony-forming units; MNC, mononuclear cells.