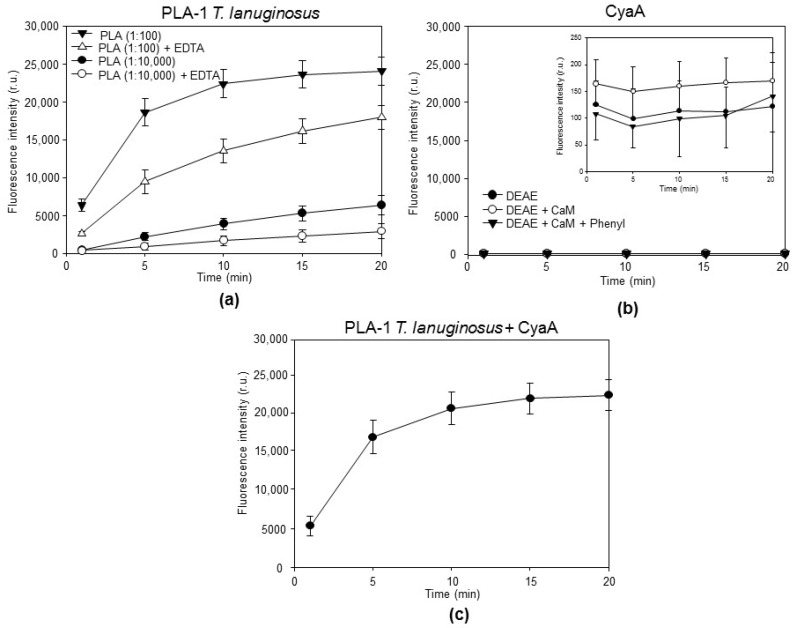
Figure 2.
The purified CyaA toxin is devoid of any detectable phospholipase A1 activity. (a) The stock solution of phospholipase A1 from Thermomyces lanuginosus was diluted 1:100 or 1:10,000 in 10 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 10 mM CaCl2 (or 20 mM EDTA), and incubated with 500 nM PED-A1, a selective PLA-1 fluorogenic substrate, before the fluorescence emission at 530 nm (excitation at 488 nm) was recorded under a continuous kinetic readout in the microtiter plate. Fluorescence intensities of the cleaved fluorogenic substrate were subtracted from the background and averaged. The values represent the average ± standard deviations derived from three independent experiments performed in triplicate (n = 9). (b) Individual purified CyaA fractions were diluted in buffer to provide a final AC enzyme activity of 1 U/mL. Incubation with 500 nM PED-A1 occurred before fluorescence emission at 530 nm was recorded at indicated time points of t = 1, 5, 10, 15, and 20 min. The values represent the average ± standard deviations derived from three independent experiments performed in triplicate with two independent CyaA preparations (n = 9). (c) The 500 nM PED-A1 substrate was mixed with highly purified CyaA (DEAE + CaM + Phenyl, 1 U/mL) and 1:100 diluted phospholipase A1 from Thermomyces lanuginosus was added. Fluorescence emission at 530 nm was recorded.