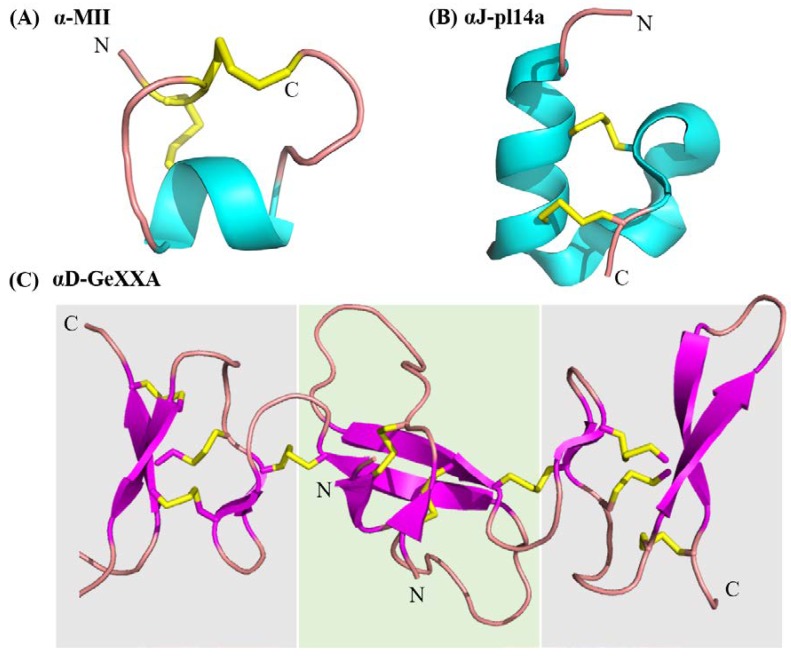
Figure 3.
Structural diversity in conotoxins modulating the nicotinic acetylcholine receptors (nAChRs). (A) α-Conotoxin MII representing a ‘typical’ globular α-conotoxin structure with a CC−C−C framework, I–III II–IV connectivity and α-helical backbone (B) αJ-pl14a—a non-classical nAChR modulator from the J superfamily, with a C−C−C−C framework, I–III II–IV connectivity resulting in a very different structure and potentially binding mode and nAChR interactions. (C) αD-GeXXA a representative of the D superfamily, which are natively dimeric modulators of nAChRs. The C-terminal domain of each monomer is shown against a grey background and the N-terminal is against a green background. Contrary to the α-helical motifs seen in other nAChR specific conotoxins, αD-GeXXA primarily consists of β-sheets. The unusual structure is also associated with a very different receptor modulation mechanism. This figure represents only a fraction of the diversity associated with conotoxin modulators of nAChRs. Table 1 provides further sequence and functional details for conotoxin modulators of nAChRs, whose structures have not been determined.