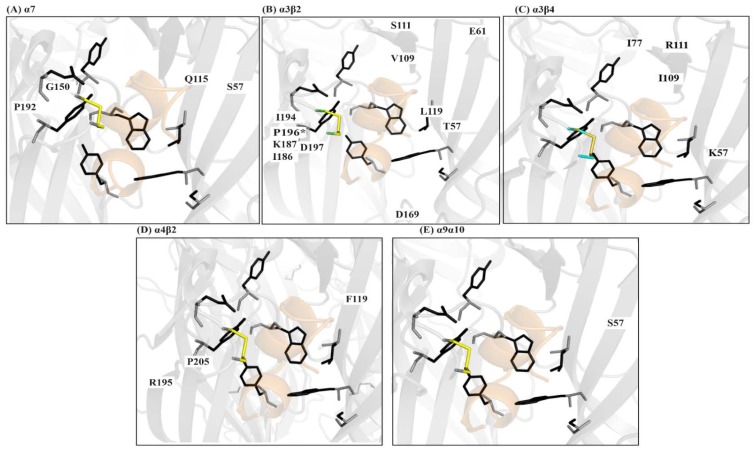
Figure 5.
nAChR selectivity determinants identified using α-conotoxins. The α-conotoxin backbone wedges deep within the conserved aromatic core in the nAChR binding pocket. The side chains extend beyond this core and engage variable residues on the (+) and (−) face of the binding pocket to obtain subtype-selectivity. A representative α-conotoxin is shown surrounded by the residues forming the aromatic core as sticks (black). Subtype selectivity determinants identified using α-conotoxins and their locations outside the core are represented by residue labels. (A) Residues modulating α7 selectivity [93,94,95,96]; (B) Residues modulating α3β2 selectivity [70,79,82,97]. P196 modulates species selectivity for the α3β2 [82]; (C) The α3β4 selectivity is largely modulated by residues in the (−) face of the binding pocket [69,84,85]; (D) Residues modulating α4β2 selectivity [79,92]; and, (E) Interactions with S57 is critical to obtain α9α10 species selectivity. α-Conotoxin interactions with this residue were key in determining the α9α10 stoichiometry as α10(+)α9(−), contrary to the previously assumed α9(+)α10(−) [98].