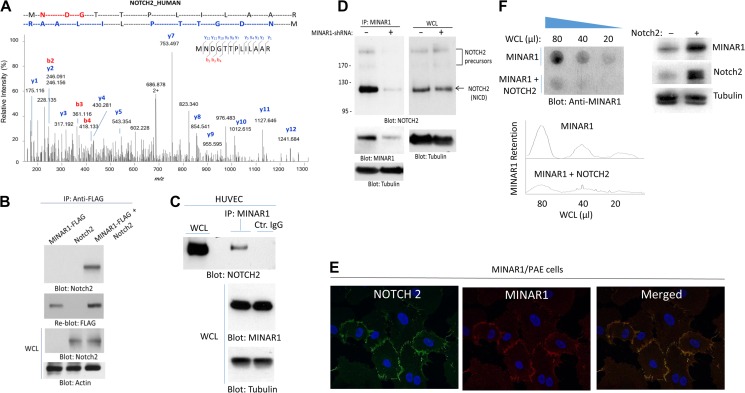
Figure 5.
Identification of Notch2 as a binding partner of MINAR1. (A) PAE cells expressing empty vector or Myc-tagged MINAR1 were lysed, immunoprecipitated with anti-Myc antibody, resolved in SDS-PAGE. The corresponding band was cut and subjected to proteolytic digestion followed by LC-MS/MS analysis of the tryptic peptides. Shown here is the MS2 higher energy collisional dissociation (HCD) spectrum corresponding to Notch2 peptide ‘MNDGTTPLILAAR’, labeled with assignments for the detected N-terminal b-ion (red) and C-terminal y-ion (blue) fragments. (B) HEK-293 cells expressing FLAG-tagged MINAR1 alone or transfected with Notch2 were lysed and immunoprecipitated with anti-FLAG antibody followed by immunoblotting with anti-Notch2 antibody. (C) Cell lysates from HUVECs were immunoprecipitated with anti-MINAR1 antibody or control IgG followed by immunoblotting with anti-Notch2 antibody. (D) HUVECs were transduced with two different MINAR1 shRNAs. After 48 h, cells were lysed and subjected to immunoprecipitation using anti-MINAR1 antibody, followed by immunoblotting with anti-Notch2 antibody. (E) PAE cells expressing MINAR1 were subjected to immunofluorescence staining for MINAR1 and Notch2. (F) Whole-cell lysates (WCL) from HEK-293 cells expressing MINAR1 alone or co-expressing MINAR1 with Notch2 were subjected to a filter trap assay as in Figure 1 and immunoblotted with anti-MINAR1 antibody. WCL of HEK-293 cells expressing MINAR1 alone or co-expressing MINAR1 with Notch2 were subjected to western blot analysis and blotted for MINAR1, Notch2, and the loading control tubulin.