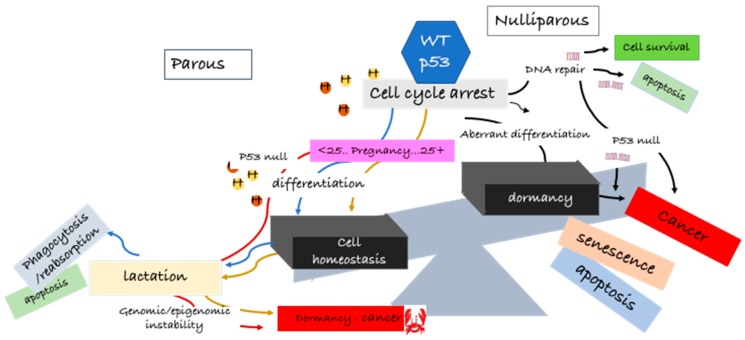
Figure 1.
A role for p53wt in breast cancer origin and latency—p53wt “good guy–bad guy” hypothesis. During pregnancy (before 25 years of age), under the influence of the female hormones (estrogen and progesterone), p53 participates in stepwise chromatin remodeling and epigenetic reprogramming. These reprogramming events imprint a lasting protective signature on mammary cells, maintaining homeostasis (blue line). After lactation, wasted cells are phagocytosed and reabsorbed or undergo apoptosis. Mammary glands in females over the age of 25 years undergo the same process; however, because of the potential genomic and epigenetic instability, resulting from the continuous pre-pregnancy hormonal flux over the years, the mammary cells are not protected against latent breast cancer. The mammary cells may remain in a pre-cancerous dormant state for decades until stimulated by mitogens to proliferate (gold line). In nulliparous females, p53null mammary cells also produce active lactating cells but are highly vulnerable to spontaneous cancer (red line). p53 tries to repair the damaged cells for survival (black line). If DNA damage cannot be repaired, cells undergo apoptosis, senescence, or remain in a pre-cancerous dormant state (black line). In aberrant differentiation, 53wt tries to repair the DNA mismatches. If unable to complete the repair, the cells undergo apoptosis or become precancerous cells (black lines). Aberrant differentiation can also lead to dormancy and, thereafter, apoptosis, senescence, or emerging of latent breast cancer cells (black line). Hypothetically, recurrence of breast cancer may follow the process of dormancy, i.e., cells do not die but remain in a vulnerable pre-cancerous state.