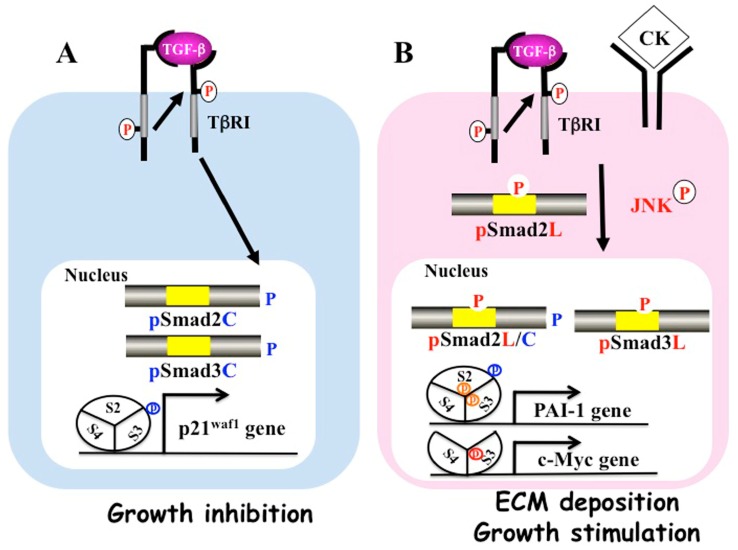
Figure 1.
Differential phospho-Smad signals between tumor suppression and fibrocarcinogenesis. (A) Activated transforming growth factor (TGF)-β type I receptor (TβRI) phosphorylates COOH-tail serine residues of Smad2 and Smad3. Both COOH-terminally phosphorylated Smad2/3 (pSmad2C and pSmad3C) translocate with Smad4 to the nuclei of quiescent hepatocytes after regeneration. Smad2/3/4, complex binds the p21waf1 promoter and suppresses cell growth; (B) Pro-inflammatory cytokines (CK) such as tumor necrosis factor-α activate c-Jun N-terminal kinase (JNK), which phosphorylates the linker regions of Smad2 and Smad3. Linker phosphorylated Smad3 (PSmad3L) translocates with Smad4 to the nucleus and binds plasminogen activator inhibitor type 1 (PAI-1) promoter. Linker phosphorylated Smad2 (PSmad2L) is localized in the cytoplasm, and Smad2 translocates to the nucleus only after COOH-tail phosphorylation by TβRI. PSmad2L/C in cooperation with pSmad3L and Smad4 stimulate PAI-1 transcription and extracellular matrix (ECM) deposition. PSmad3L up-regulates c-Myc and stimulates cell growth, while suppressing the pSmad3C-mediated tumor suppressive pathway.