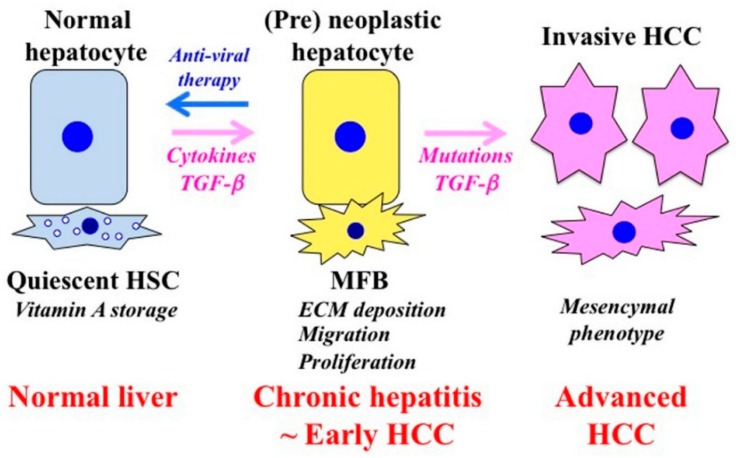
Figure 2.
Phenotypic alternations of hepatocytes and HSC during the fibrocarcinogenic process in human chronic liver diseases. Quiescent hepatic stellate cells (HSC) are characterized by retinoid droplets in the cytoplasm and maintain liver homeostasis. HSC undergo constitutive activation to become myofibroblasts (MFB)-like cells after liver injury. MFB persistently produce an extracellular matrix (ECM) and induce liver fibrosis. The contraction of MFB contributes to increased portal resistance during liver fibrosis that presumably is reversible until the thickened septae, intrahepatic shunts, and lobular distortion that are characteristic of cirrhosis development, leading to fixed increases in portal pressure. Chronic liver damage promotes recurrent cycles of cellular proliferation, inflammation, fibrosis, and carcinogenesis. In pre-neoplastic hepatocytes, several growth factors and cytokines activate proliferation and invasion. As human hepatitis virus-related chronic liver diseases progress, chronic inflammation and hepatitis virus additively accelerate liver fibrosis and increase the risk of hepatocellular carcinoma (HCC). Genetic and epigenetic changes in the liver result in carcinogenesis. Effective antiviral therapy can reverse the pre-neoplastic properties of hepatocytes to a tumor-suppressive mode before the occurrence of genetic mutations that have been implicated for HCC occurrence.