1. Introduction
The lateral hypothalamus (LH) is part of a descending system that modifies nociception in the spinal cord dorsal horn. Both electrical (Lopez and Cox,1992; Dafny et al.,1996; Franco and Prado, 1996) and chemical stimulation with S-glutamate (Behbehani et al., 1988), morphine (Dafny et al., 1996; Franco and Prado, 1996), carbamylcholine (carbachol; Holden & Naleway, 2001; Holden et al., 2002; Holden et al., 2005; Holden and Pizzi, 2008; Holden et al., 2009; Safari et al., 2009), or nociception/orphanin FQ (Gerashchenko et al., 2011;) increase response latencies to acute (nociceptive) pain in rats. This nociceptive modulation was found in both male (Dafny et al., 1996; Franco and Prado, 1996; Behbehani et al., 1988; Safari et al., 2009; Gerashchenko et al., 2011) and female rats (Lopez and Cox, 1992; Holden and Naleway, 2001; Holden et al., 2002; Holden et al., 2005; Holden and Pizzi, 2008; Holden et al., 2009). The role of the LH in modulating neuropathic pain states is not known for either male or female rats. However, there is evidence that neuropathic pain, including hyperalgesic pain states, creates a shift from opioid mediated responses to noradrenergic-mediated mechanisms (Hartrick, 2012; Rojo et al., 2012; Schiene et al., 2011; Schroder et al., 2010). This finding is in line with recent reports from our laboratory and others that the LH modifies nociception in the spinal cord dorsal horn in part through descending alpha adrenergic brainstem neurons (Holden and Naleway, 2001; Holden et al., 2002; Holden et al., 2005; Holden and Pizzi 2008; Holden et al., 2009; Safari et al., 2009) and supports the hypothesis that LH stimulation will modify both nociceptive and neuropathic pain in male and female rats.
The purpose of this study was to determine the effect of three different doses of carbachol stimulation of the LH on paw withdrawal responses in male and female Sprague-Dawley rats that received one of two pain conditions: neuropathic pain from chronic constriction injury (CCI), or naïve rats in which the thermal stimulus was analogous to acute, or nociceptive, pain (Loeser and Treede, 2008; Xu et al., 2012). The CCI model is a valid and reliable method of producing thermal hyperalgesia, one of the symptoms of neuropathic pain (Bennett and Xie, 1988; Bennett, 1993; Atal et al., 1990; Kim et al., 1997; Jeong and Holden, 2009). The paw withdrawal latency (PWL) was used to test responses to a thermal stimulus and has proven reliability and validity (Yeomans and Proudfit, 1994). A preliminary account of these results has been published as an abstract (Wagner, et al., 2012).
2. Experimental Procedures
The Institutional Animal Care Committee at the University of Michigan approved the experimental protocols used in this study. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). All efforts were made to minimize animal suffering, reduce the numbers of animals used, and use alternatives to in vivo experiments.
Animals
Male and female Sasco-derived Sprague-Dawley rats (250–350 g; Charles River, Portage, MI) were used. All rats were maintained on a 12-hour day/night schedule with free access to food and water. To reduce the possibility of estrous cycle influence, female rats were randomly assigned to either experimental or control groups and no two rats were taken from the same cage on the same day. To reduce the risk that mirror image effects on the non-ligated paw may be occurring, (Kim et al., 1997) we used separate control animals rather than having each animal serve as its own control. Two hundred ninety one rats were used for the study as reported, and each rat was used only once.
CCI procedure
Under isoflurane anesthesia, the left thigh of the rat was infused with bupivacaine (0.5%; 0.10 ml). The common sciatic nerve was exposed at the level of the mid-thigh by blunt dissection through the biceps femoris. Proximal to the sciatic trifurcation, about seven mm of nerve was freed of adhering tissue and four ligatures (4.0 chromic gut) were tied loosely around the nerve, about 1 mm apart. The length of the nerve affected was 4 to 5 mm long. The ligatures were tied to lightly constrict the diameter of the nerves when viewed with 40X magnification, and standardized by observing for the initial twitching of the paw as the ligature was tightened. The muscle was sutured with 4.0 chromic gut and the incision closed with wound clips. Each rat received a subcutaneous injection of buprenorphine (0.3 mg/ml) at a dose of 0.05 mg/kg, recovered and returned to its cage.
Analgesiometric testing procedures
To determine the effect of LH stimulation on thermal nociception, the PWL was used. The paw was exposed to a focused beam of radiant heat using an analgesiometer (37360, Ugo Basile, Italy). Using a heat flux radiometer, the radiant beam of each machine was adjusted to a maximum intensity of 130 mW/cm2. The time interval between the onset of skin heating and the withdrawal response was measured electronically. In the absence of a response, skin heating was terminated after 20 sec to prevent burning. Paw response latencies were measured at one place on the hairy surface of each hind paw. Baseline response latencies were approximately 6–8 sec. Temperature was measured with a rectal probe pre-injection, then at 5 min post injection and every 10 min thereafter. Heart rate, blood pressure and mean arterial pressure were measured preinjection and following the final latency measurement using a tail cuff and Coda monitor (Kent Scientific; Torrington, CT)
Estrodial/progesterone measurement
After thermal testing, cardiac puncture was used to draw blood for determining the serum estradiol/progesterone ratio in females to use for covariate analysis if indicated. Following completion of analgesic testing, female rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, IP) and the chest opened. Using a 26 g needle attached to a 3 cc syringe, the cardiac ventricle was punctured and 1.5 cc blood withdrawn. The blood was then injected into a serum separator tube, gently inverted 2–3 times and left to sit for a 30–60 min. The tube was then spun on a micro-centrifuge for 15 min at 3100 rpm, and refrigerated at −70° C until a batch was ready to send for analysis (Antech Diagnostics; Morrisville, NC).
Experiment 1: Nociceptive responses in CCI vs. non-CCI rats
To demonstrate that CCI ligation produced thermal hyperalgesia, male and female rats received CCI ligation of the left leg or sham surgery for control. In sham surgery, the sciatic nerve was exposed to the air but not ligated. Two weeks later each rat was lightly anesthetized with sodium pentobarbital (35 mg/kg, IP). Response latencies were then measured at 1 min and then every 5 min for 45 min.
Experiment 2: Carbachol or saline microinjection in the LH
Male and female rats were randomly assigned to pain condition (nociceptive or neuropathic) and to carbachol dose (125, 250, 500 nmol in 0.5 μl normal saline). Rats in the neuropathic group received CCI ligation, as described above, two weeks prior to Experiment 2. Rats in the nociceptive group did not receive ligation. Each rat was lightly anesthetized as described above and the scalp infused with the local anesthetic lidocaine (1%; 0.15 ml). Supplemental doses of pentobarbital were given during the procedure if the rats vocalized or moved without stimulation, but were rarely required. Immediately after anesthesia, the midline scalp was shaved and the rats were immobilized in a stereotactic apparatus. Using aseptic technique, a 2-cm incision was made, and the muscle and fascia retracted. A 23-gauge stainless steel guide cannula was lowered through a burr hole into the region of the left LH defined by the following stereotactic coordinates: AP −1.5 mm from bregma, lateral +1.6 mm, vertical +2.2 mm, incisor bar set at −2.5 mm. A 30-gauge stainless steel injection cannula was connected to a 10 μl syringe by a length of PE-10 polyethylene tubing filled with either saline or a solution of carbachol of either 125, 250 or 500 nmol in normal saline injected in a volume of 0.5 μl (Sigma Chemical Co., St. Louis, MO; All solutions were made fresh daily and filtered through a 0.2 μm syringe prior to use). The injection cannula was then inserted and extended approximately 3 mm beyond the end of the guide cannula. After a baseline response latency measurement was taken and recorded, either saline or carbachol was injected into the LH over a 1-min period using an electronic syringe pump. The injection cannula was left in place for an additional 60 sec following completion of the microinjection to reduce flow of drug up the guide cannula. Response latencies were then measured at 1 min post injection and then at 5 min intervals until a return to baseline or for 45 min.
Histology
Following testing, animals were overdosed with sodium pentobarbital and decapitated. The brains were taken and drop fixed in a solution of 10% neutral-buffered formalin. To determine the position of the microinjection sites relative to the LH, 40-μm transverse brain sections were cut from blocks of tissue that contained the visible injection cannula tract using a cryostat microtome. The sections were rinsed in cold phosphate-buffered saline PBS (10 mM), mounted on gel-coated slides, stained with 0.05% neutral red, dehydrated through a series of alcohols and xylenes, and cover slipped. The placement of the microinjection cannula was determined by locating the most ventral position of the cannula tip in serial sections by brightfield microscopy. Tracings of the appropriate sections were then made using the Neurolucida imaging system (Microbrightfield, Colchester, VT). The tracings were compared with drawings from the atlas of Paxinos and Watson (2009) to verify that the cannula was within the LH.
Statistical analysis
Treatment groups consisted of between 11 and 16 rats. Paw withdrawal latencies are presented as the mean ± S.E.M. For Experiment 1, statistical comparisons among treatment groups across multiple time points were made using two-way repeated measures ANOVA, and comparisons among means at specific time points were made using the Bonferroni t-test for multiple post-hoc comparisons using Sigma Stat 3.5 (Systat Software, Inc, Point Richmond, CA). In addition, paired t-test was used to compare withdrawal latencies between left and right paws for both male and female rats.
The analysis for Experiment 2 was done using the generalized linear mixed models (GLMM) with pain condition, sex, carbachol dose, and interactions entered as between-subjects factors, and time as a within-subjects factor. Post hoc contrast tests were conducted, adjusting for Bonferroni correction among different time-points. Data analysis was performed using SAS 9.2 statistical software (SAS Inc., Cary, NC).
3. Results
Microinjector placement
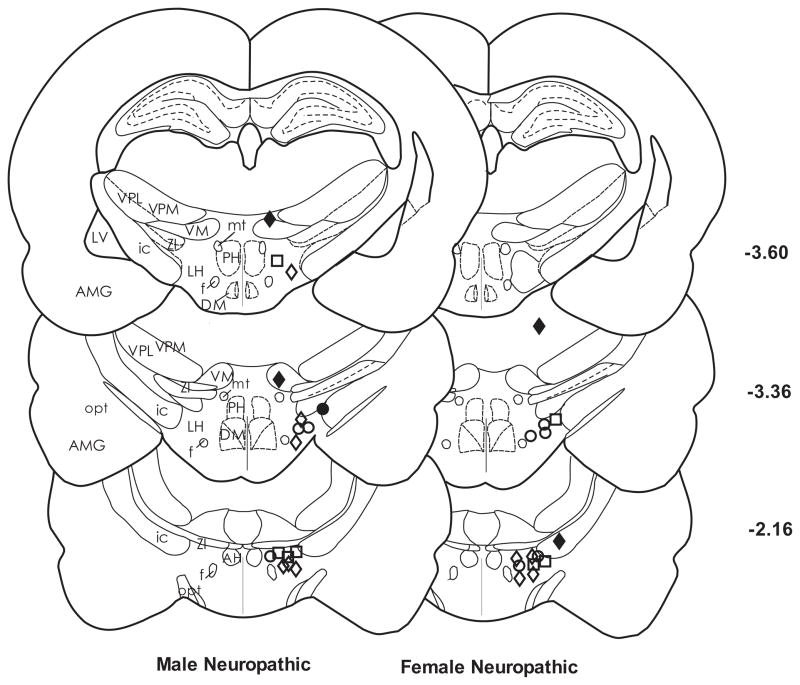
Figure 1 is a representative drawing of microinjector placement for male and female CCI groups that received 500 nmol of carbachol, the dose that produced the most consistent antinociceptive effect. Microinjection sites for the nociceptive males and females were similar (not shown). Most of the injection sites were located within the area defined as the LH (Paxinos and Watson, 2009). Microinjections of carbachol outside of the LH in the ventral thalamus, zona incerta and amygdala produced some latency change for nociception. Therefore, data were analyzed only for microinjections within the LH.
Figure 1.
Representative location of microinjection sites in the LH for left PWL for the 500 nmol dose of carbachol in CCI rats. Most of the injection sites were located within the border of the LH between AP −2.12 and −3.60 mm from bregma. The symbols represent the differences between baseline paw response latencies and those at the peak time of carbachol effectiveness (10 min for females and 20 min for males). Symbols for response latency after microinjection of carbachol are as follows: (◇) 3–8 sec; (○) 9–14 sec; (□) 15–20 sec. Injection sites located in the zona incerta and amygdala (●;■) and the ventral thalamus or optic tract (◆) were excluded from data analysis. AH, anterior hypothalamic area; AMG, amygdala; ic, internal capsule; LH, lateral hypothalamus; LV, lateral ventricle; mt, mammillothalamic tract; opt, optic tract; PH, posterior hypothalamus; VM, ventromedial thalamic nucleus; VPL, ventral posterolateral thalamic nucleus; VPM; ventral posteromedial thalamic nucleus; ZI, zona incerta.
Effect of CCI ligation on male and female rats
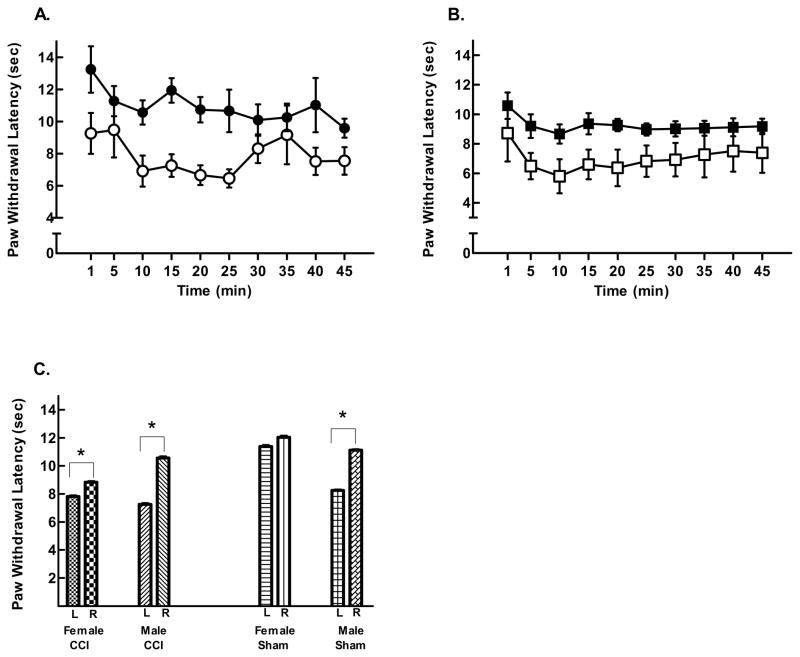
CCI ligation produced significant hyperalgesia on the left paw of both male and female rats as shown by faster withdrawal latencies compared to sham surgery control rats. Figure 2A shows that male CCI rats withdrew their left paw significantly faster than control rats (two way repeated measures ANOVA, F = 4.695(1, 187); p = 0.04; 7.26 ± 0.65 vs. 9.27 ± 0.65 respectively). Female CCI rats also withdrew their left paw from the heat stimulus faster than sham control rats (two way repeated measures ANOVA, F = 8.712(1, 234); p = 0.007; 7.82 ± 0.85 vs. 11.39 ± 0.85 sec respectively; Fig. 2B)
Figure 2.
CCI ligation produced decreases in left PWL. (A) Female CCI rats (○) had significantly shorter paw withdrawal latencies than sham surgery controls (●). (B) Male CCI rats also had significantly shorter withdrawal latencies (□) compared to sham controls (■). (C) Both female and male CCI rats demonstrated shorter average PWL on the left (L) compared to the right (R) paw. However, while female sham surgery control rats showed no left/right paw differences, males sham surgery controls had significantly shorter left PWL compared to right paws (averaged response latencies at 10 min post baseline). Mean response latencies ± SEM plotted on the ordinate as a function of time; n = 14 per group (*p < 0.05).
Because the difference between male CCI rats and their controls was smaller than females and their controls, comparisons were made between the left and right paw withdrawal responses within groups. Figure 2C shows significant differences between the left and right paw withdrawal latencies in CCI male (Student’s T-test; 7.26 ± 0.35 vs 10.57 ± 0.45 sec; p < 0.001; left and right resp.) and female rats (7.82 ± 0.34 vs. 8.84 ± 0.30; p =0.025). Interestingly, in sham surgery control rats, females showed no significant differences for left and right withdrawal latencies (p > 0.05) as was expected, but male sham rats demonstrated significant left (8.26 ± 0.22 sec) vs. right (11.13 ± 0.3 sec; p < 0.001) differences, indicating that the surgery itself produced hyperalgesia in males in the absence of sciatic nerve ligation. However, this hyperalgesia was less than that produced by CCI ligation.
Effect of sex, pain condition and carbachol dose on male and female rats
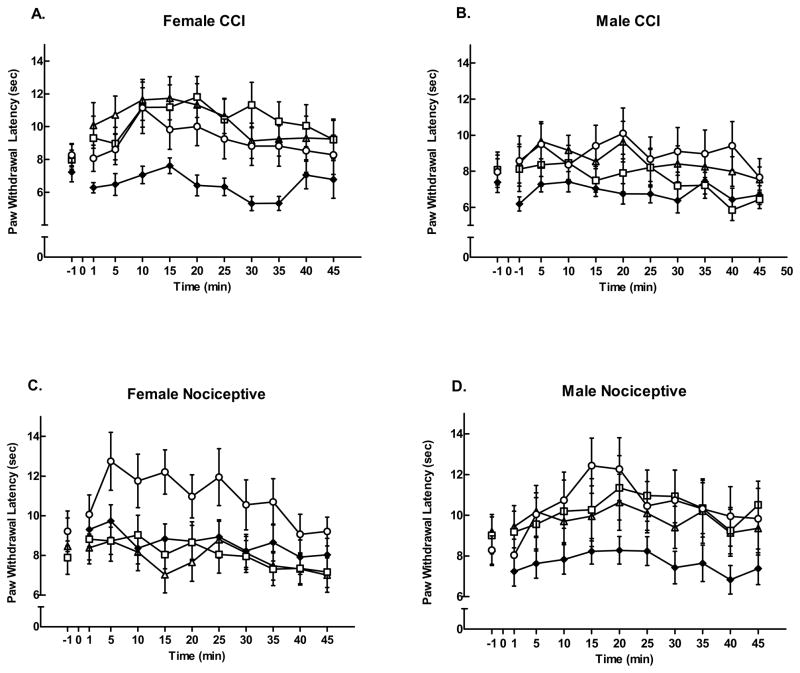
Male and female rats in both CCI and nociceptive groups demonstrated significant antinociception (time effect, F = 9.70, p = 0.0019) for left PWL following LH stimulation (Figure 3). There was a significant dose effect (F = 4.31, p = 0.005). In general, the highest carbachol dose (500 nmol) produced the greatest effect across all groups compared with control rats (p < 0.05). Male and female rats responded differently by pain condition and carbachol dose across time (significant gender*group*dose*time effect, F = 2.95, p = 0.032). Female CCI rats (Fig. 3A) showed equivalent responses to the three carbachol doses, while male CCI rats (Fig. 3B) showed more variability for dose and a smaller response to LH stimulation than females. However, in nociceptive pain, females responded only to the 500 nmol dose (Fig. 3C), while males responded to all doses (Fig. 3D).
Figure 3.
Microinjection of carbachol in the left LH produced dose-dependent increases in left PWL. Following a baseline response latency measurement at time −1 min, normal saline (◆) or carbachol in one of three doses: 125 nmol (△); 250 nmol (□); or 500 nmol (○) was microinjected into the LH at time 0. The 500 nmol dose was the most effective across groups compared to saline control rats, but dose response was dependent on sex of the rat and pain condition. Female CCI rats (A) showed equivalent responses to the three carbachol doses, while male CCI rats (B) showed more variability for dose and a smaller, but significant, response to LH stimulation than females. However, nociceptive females responded only to the 500 nmol dose (C), while nociceptive males responded to all carbachol doses (D). Mean response latencies ± SEM are plotted on the ordinate as a function of time (min).
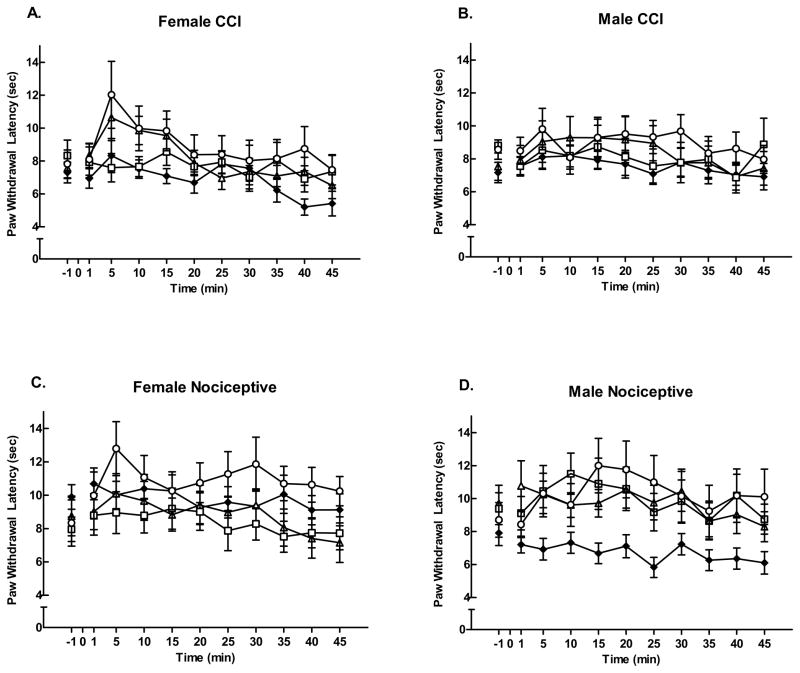
In contrast, data for right PWL (Figure 4) illustrated less response variability between males and females by dose and pain condition (insignificant gender x group x dose x time effect, F = 0.16, p = 0.92). Overall there were no notable dose effects, except that nociceptive males remained likely to respond to all doses in acute pain (Fig. 4D).
Figure 4.
Effects of carbachol microinjection in the left LH on right PWLs. Neither CCI group (A and B) showed a significant right paw effect at any dose of carbachol (125 nmol (△); 250 nmol (□); or 500 nmol (○), compared to saline for control (◆), nor did female nociceptive rats (C). Male nociceptive rats (D) demonstrated a significant increase in PWL at all doses of carbachol compared to the saline control group. Mean response latencies ± SEM plotted on the ordinate as a function of time (min).
Estrous cycle
Stage of estrous cycle was determined as a ratio of estradiol to progesterone values and compared to the table found in Butcher et al. (1974). Estrous cycle stage was variable across the four female groups and there was not a sufficiently large number per stage of estrous per group to analyze for covariance.
Temperature, heart rate, and blood pressure
Baseline data for temperature, heart rate, and blood pressure were similar among different experimental groups. There was no notable difference in temporal patterns of temperature data between male and female rats; left and right paw; CCI and nociceptive groups; or among various dose groups. The variability of temperature across time among different subgroups was negligible, mostly within one Fahrenheit degree. Heart rate and blood pressure data illustrated similar results, and no significant differences in delta change among subgroups were observed (data not shown).
4. Discussion
Findings from the present study are suggestive of differences in descending modulation based on sex, carbachol dose, and pain condition of rats; stimulating the LH induced a significant antinociceptive effect on thermal PWLs in both male and female CCI rats with thermal hyperalgesia as well as nociceptive rats, in which the thermal stimulus was analogous to acute pain. All three carbachol doses were effective for female CCI rats, and to a lesser extent, for male CCI rats. In nociceptive rats, only the 500 nmol dose was effective for females while males responded to all three doses.
Descending modulation is inherent in the normal processing of pain, acting through both inhibition and facilitation mechanisms (Millan, 2002; Ossipov et al., 2010). Descending modulation is altered in neuropathic pain (Vanegas and Schaible, 2004) including such conditions as irritable bowel syndrome (Bannister et al., 2009; Heymen et al., 2010), temporal mandibular disorder (Bannister K et al., 2009), complex regional pain syndrome (Seifert et al., 2009), chronic pancreatitis (Olesson et al., 2010), tension headache (Pielsticker et al., 2005) and fibromyalgia (Lautenbacher and Rollman, 1997; Normand et al., 2011). The findings of the present study indicated that descending modulation is induced by LH stimulation in both neuropathic and nociceptive pain conditions. The reason for the effectiveness of LH-induced antinociception may relate in part to connections of the LH to descending brainstem systems. Several studies indicate that the periaqueductal gray (PAG) and the rostral ventromedial medulla (RVM) constitute the outflow of descending modulation and that peripheral damage can alter the balance between inhibition and facilitation of nociception by these brainstem structures (Niesters et al., 2013; De Felice et al., 2011; Heinricher et al., 2009; Vanegas and Schaible, 2004). The LH sends projections to both the PAG (Clement et al., 2012; Hsieh et al., 2011; Holden et al., 2009) and RVM (Barba, et al., 2014; Holden et. al., 2005; Herman, et al., 1997) as well as the descending noradrenergic neurons in the dorsolateral pontine tegmentum (Tokita et al., 2009; Holden and Naleway, 2000; Holden, et al., 2001). Stimulation of the LH produces both inhibition and facilitation through these structures in a nociceptive model of pain in females. Disruption of any one of these areas with cobalt chloride which reversibly blocks all synaptic activity in the area of microinjection (Barnabi and Cechetto, 2001; Kretz, 1984; Norton et.al., 1985) eliminates LH-induced antinociception, suggesting that effective descending modulation is a network of structures that produces summation of facilitation and inhibitory effects that favors inhibition.
Sex differences in pain responses and modulation have been found in humans and animals, although a recent systematic review of the literature could not demonstrate a consistent pattern of gender differences in responses to pain in humans (Racine et al., 2012). Generally speaking, women report greater pain, are at higher risk of developing clinical pain states, and require higher doses of morphine to relieve pain than men (Mylius et al., 2005; Aubrun et al., 2005; Fillingim et al., 2009), and have lower tolerance for pressure and thermal pain, but have responses comparable to men for cold and ischemic pain (Racine et al., 2012). Female rats experience more pronounced low dose morphine hyperalgesia than males (Holtman and Wala, 2005), develop complete Freund’s adjuvant-induced inflammation faster than males, exhibit greater hyperalgesic effects, and require higher doses of morphine and oxycodone than males (Cook and Nickerson, 2005). Antinociception is also significantly greater in male than female rats overall on the tail flick and electric shock-induced jump tests following intrathecal injection of clonidine, an alpha2-adrenoceptor agonist (Kiefel and Bodnar, 1991). Additionally, females exhibit long term mechanical hyperalgesia compared to male rats at several lower volumes of hypertonic saline injected into muscle, while males show significantly longer thermal paw withdrawal latencies at a higher volume (0.4ml) than females (Lei et al., 2011). Findings from the current study support and extend these earlier findings of sex differences in both acute and hyperalgesic models of nociception, with the notable finding that female antinociceptive responses, particularly in the hyperalgesic model, are greater than those of males.
Previous work from this laboratory demonstrated that the 125 nmol dose of carbachol was effective in reducing nociceptive pain in female rats, a finding contrary to the findings of the present study. This difference may be attributed to a different heat source. In the previous study we used a projection bulb suspended over the paw that heated an area of skin approximately 5 mm in diameter with intense light, which was encircled by a penumbra of less intense light. In the current study the size of the thermal heat stimulus was approximately 2mm. Differences in recruitment and summation of peripheral fibers likely contributed to the different dose responses.
An unexpected finding was that male sham surgery rats showed significant hyperalgesia of the left compared to the right paw two weeks post incision. Given that sham surgery is an acute procedure, we expected that neither sham group would show left-right paw differences. It is possible that this finding is due to technical error, but that explanation is unlikely. A single technician did the CCI and sham surgeries, and females showed no significant left right differences. In male rats, persistent secondary hyperalgesia has been observed following incision of the gastrocnemius muscle due to hypoxia and elevated lactate and pH levels at the incision site that could sensitize nociceptors, and increase central sensitization (Kang et al., 2013; Kim et al., 2007; Woo et al., 2004; Pogatzki et al., 2002). This finding is in line with the finding of the present study with male rats, but the reason for a lack of hyperalgesia in sham females is not clear. One explanation for male sham differences may relate to thermosensitive TRPV channels that are expressed in sensory neurons and that detect distinct temperature thresholds in both humans and mice (Smith et al., 2002; Moqrich et al., 2005; see Dhaka et al., 2006, and Schepers and Ringcamp, 2010 for review). Recent ongoing work showed that male rats had a greater preference and sensitivity for temperature differences compared to females, possibly due to differences in sensitivity of TRP channels (Carstens et al., 2013). Whether this difference is observable in lightly anesthetized rats as in the current study is not clear, but the presence of male-female differences in TRP channels is an avenue worth exploring.
Early electrolytic lesion work implicates the LH in temperature regulation (Arase et al., 1987; Monda et al., 1996; Refinetti and Carlisle, 1986; 1987). More recent studies stress the role of the preoptic, paraventricular and dorsomedial hypothalamic areas in temperature control (Osaka, 2012, 2009, 2004; Morrison and Nakamura, 2011; Nakamura, 2011; de Menezes et al., 2009; Nakamura and Morrison, 2007; Nagashima et al., 2000). However, orexin-containing neurons in the LH perifornical area are now known to affect brown fat tissue (BAT) thermogenesis (Tupone et al., 2011; Richard, 2007; Oldfield et al., 2002), but only when body temperature is maintained below 37° (Morrison et al., 2012; Tupone et al., 2011). In our experiments, it is likely that we stimulated orexin neurons, but as the body temperatures of our rats were maintained at 37° or above, the likelihood that nociceptive responses from LH stimulation were from changes in temperature is small.
The LH has also been identified as a contributor to cardiovascular regulation, including decreasing (Smith et al., 2007; Brown et al., 2007), or increasing blood pressure (Shahid et al., 2012; Xiao et al., 2013). Because group blood pressure and heart rate stayed within a reasonable physiological range during the testing of our rats, it is unlikely that cardiovascular effects acted as a confounding variable for our findings.
A limitation of microinjector studies is the difficulty in determining the precise microinjection size. Previous work has shown that molecular weight, concentration (Myers and Hoch, 1978; Nicholson, 1985) and water solubility of the solution, injector tip size (Sakai et al., 1979) and neuroanatomical features of the injection site (Nicholson, 1985; Sakai et al., 1979; Lum et al., 1984) can all affect spread of the injected solution. Generally speaking, microinjections of 0.5 μl or less limit the average spread of injection (Myers and Hoch, 1978). While recent findings indicate that a 0.5 μl injection of [3H]muscimol into subcortical structures produces a labeled area of over 5 mm2 15 minutes after injection (Edeline et al., 2002), others have reported smaller areas of spread, with average injections sites of 1–1.5 mm within the first 20 min post injection (Grossman and Stumpf, 1969; Myers and Hoch, 1978; Martin, 1991). Our microinjections of 0.5 μl likely fall within these parameters. It is also important to note that the amount of available solution relative to the injection location is considerably lower the farther away from the injector tip (Myers and Hoch, 1978; Grossman and Stumpf, 1969). Similarly, diffusion of a solution does necessarily equate with neuron activation. Microinjection of carbachol into the ventral thalamus just dorsal to the LH (Holden and Pizzi, 2009), the nigrostriatal bundle (Holden et al., 2002) and the internal capsule adjacent to the LH (Holden et al., 2005; Holden et al., 2002) produces withdrawal latencies similar to baseline measurements. Finally, we showed previously that 62 nmol of carbachol produces response latencies similar to control rats when microinjected into the LH (Holden and Naleway, 2001). Given our use of a small microinjector tip, exclusion of injections outside the LH, the evidence that neurons closer to the microinjector tip are more likely to be activated than neurons farther away, and the lack of response from a smaller dose of carbachol, the probability that our results came from stimulating neurons outside of the LH is small.
In the present study, unilateral microinjection of carbachol did not produce significant differences on right paw withdrawal latencies except for male nociceptive rats. The LH projection to the spinal cord is primarily ipsilateral (Kuypers and Maisky, 1975; Hancock, 1976; Saper et al., 1976; Hosoya, 1980) and our findings support this observation. The reason for right paw responses to LH stimulation in male naïve rats is unclear. However it is notable that right paw withdrawal response for saline control rats in nociceptive males was almost two seconds faster than the left paw and this difference may represent random variability.
In summary, the results of the present study demonstrated that microinjection of carbachol in the LH produced antinociception for female and male rats with both hyperalgesic and nociceptive pain with no significant effects on temperature or cardiovascular responses. These preclinical findings are suggestive that LH stimulation may be effective in reducing both neuropathic and nociceptive pain in humans, but responses may differ based on sex, pain condition and dose of stimulation.
Highlights.
Male and female rats showed thermal hyperalgesia from left paw CCI.
Carbachol in the LH produced antinociception in males and females on the left paw.
Carbachol dose responses differed based on sex of the rat and pain condition.
Acknowledgments
This work is supported by USPHS grant NR04778 from the National Institute of Nursing Research (NINR) at the National Institutes of Health. NINR had no involvement in the conduct of this study, or in publication decisions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arase K, Sakaguchi T, Bray GA. Lateral hypothalamic lesions and activity of sympathetic nervous system. Life Sci. 1987;41:657–662. doi: 10.1016/0024-3205(87)90421-8. [DOI] [PubMed] [Google Scholar]
- Attal N, Jazat F, Kayser V, Guibaud G. Further evidence for pain-related behaviors in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- Bannister K, Bee LA, Dickenson AH. Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics. 2009;6:703–712. doi: 10.1016/j.nurt.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Acute exercise-induced activation of Phox2b-expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience. 2014;258:355–363. doi: 10.1016/j.neuroscience.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Barnabi F, Cechetto DF. Neurotransmitters in the thalamus relaying visceral input to the insular cortex in the rat. Am J Physiol Reg, Integra Comp Physiol. 2001;281:R1665–R1674. doi: 10.1152/ajpregu.2001.281.5.R1665. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Park MR, Clement ME. Interactions between the lateral hypothalamus and the periaqueductal gray. J Neurosci. 1988;8:2780–2787. doi: 10.1523/JNEUROSCI.08-08-02780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. An animal model of neuropathic pain: A review. Muscle & Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brown SN, Chitravanshi VC, Kawabe K, Sapru HN. Microinjections of melanin concentrating hormone into the nucleus tractus solitarius of the rat elicit depressor and bradycardic responses. Neuroscience. 2007;150:796–806. doi: 10.1016/j.neuroscience.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Carstens MI, Huebner P, Nishida K, Trannguyen M, Carstens E. Neuroscience Meeting Planner, Program No. 369.1/FFF17. San Diego, CA: Society for Neurosciene. Online; 2013. Thermal discrimination of innocuous and noxious temperatures in rats. [Google Scholar]
- Clément O, Sapin E, Libourel PA, Arthaud S, Brischoux F, Fort P, Luppi PH. The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci. 2012;32:16763–16774. doi: 10.1523/JNEUROSCI.1885-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Nickerson M. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s Adjuvant-induced arthritic male and female rats. J Pharm Exp Thera. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Craft R, Lee D. NMDA antagonist modulation of morphine antinociception in female vs. male rats. Pharmacol Biochem Be. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Dafny N, Dong WQ, Prieto-Gomez C, Reyes-Vasquez J, Stanford, Qiao JT. Lateral hypothalamus: Site involved in pain modulation. Neuroscience. 1996;70:449–460. doi: 10.1016/0306-4522(95)00358-4. [DOI] [PubMed] [Google Scholar]
- De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields H, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Cardiovascular and thermal responses evoked from periaqueductal grey require neuronal activity in the hypothalamus. J Physol. 2009;587:1201–1215. doi: 10.1113/jphysiol.2008.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Sarkar S, Fontes MA, Dimicco JA. Microinjection of muscimol into the periaqueductal gray suppresses cardiovascular and neuroendocrine response to air jet stress in conscious rats. Am J Physiol Reg-I. 2008;295:R881–R890. doi: 10.1152/ajpregu.00181.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: A reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002;78(1):100–124. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;5:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco ACJ, Prado WA. Antinociceptive effects of stimulation of discrete sites in the rat hypothalamus: Evidence for the participation of the lateral hypothalamus area in descending pain suppression mechanisms. Brazilian J Med Biol Res. 1996;29:1531–1541. [PubMed] [Google Scholar]
- Geraschenko D, Horvath TL, Xie X. Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociception/orphanin FQ blocks stress-induced analgesia. Neuropharmacology. 2011;60:543–549. doi: 10.1016/j.neuropharm.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SP, Stumpf WE. Intracranial drug implants: An autoradiographic analysis of diffusion. Science. 1969;166(3911):1410–1412. doi: 10.1126/science.166.3911.1410. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Cells of origin of hypothalamo-spinal projections in the rat. Neurosci Lett. 1976;3:179–184. doi: 10.1016/0304-3940(76)90070-7. [DOI] [PubMed] [Google Scholar]
- Hartrick CT. Noradrenergic reuptake inhibition in the treatment of pain. Expert Opin Investig Drugs. 2012;21:1–8. doi: 10.1517/13543784.2012.731393. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain. 2010;26:104–109. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J, Naleway E, Jeong Y. Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience. 2005;135:1255–1268. doi: 10.1016/j.neuroscience.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Holden J, Pizzi J. Lateral hypothalamic-induced antinociception may be mediated by a substance P connection with the rostral ventromedial medulla. Brain Res. 2008;1214:40–49. doi: 10.1016/j.brainres.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J, Pizzi J, Jeong Y. An NK1 receptor antagonist microinjected into the periaqueductal gray blocks lateral hypothalamic-induced antinociception in rats. Neurosci Lett. 2009;453:115–119. doi: 10.1016/j.neulet.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Naleway E. Microinjection of carbachol in the lateral hypothalamus produces opposing actions on nociception mediated by α1- and α2-adrenoceptors. Brain Res. 2001;991:27–36. doi: 10.1016/s0006-8993(01)02567-7. [DOI] [PubMed] [Google Scholar]
- Holden JE, Schwartz E, Proudfit H. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by α1- and α2-adrenoceptors. Neuroscience. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- Holden JE, Van Poppel A, Thomas S. Antinociception from lateral hypothalamic stimulation may be mediated by NK1 receptors in the A7 catecholamine cell group in rat. Brain Res. 2002;953:195–204. doi: 10.1016/s0006-8993(02)03285-7. [DOI] [PubMed] [Google Scholar]
- Holtman J, Wala E. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Hosoya Y. The distribution of spinal projection neurons in the hypothalamus of the rat, studied with the HRP method. Exp Brain Res. 1980;40:79–87. doi: 10.1007/BF00236665. [DOI] [PubMed] [Google Scholar]
- Hsieh KC, Gvilia I, Kumar S, Uschakov A, McGinty D, Alam MN, Szymusiak R. c-Fos expression in neurons projecting from the preoptic and lateral hypothalamic areas to the ventrolateral periaqueductal gray in relation to sleep states. Neuroscience. 2011;188:55–67. doi: 10.1016/j.neuroscience.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Holden J. The role of spinal orexin-1 receptors in posterior hypothalamic modulation of neuropathic pain. Neuroscience. 2009;159:1414–1421. doi: 10.1016/j.neuroscience.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Lee D, Theusch BE, Arpey CJ, Brennan TJ. Wound hypoxia in deep tissue after incision in rats. Wound Repair Regen. 2013;21:730–739. doi: 10.1111/wrr.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kretz R. Local cobalt injection: A method to discriminate presynaptic axonal from postsynaptic neuronal activity. J Neurosci Meth. 1984;11:129–135. doi: 10.1016/0165-0270(84)90030-x. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Maisky VA. Retrograde axonal transport of horseradish peroxidase from spinal cord to brain stem cell groups in the cat. Neurosci Lett. 1975;1:9–14. doi: 10.1016/0304-3940(75)90004-x. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Lei J, Jin L, Zhao Y, Sui M-Y, Huang L, Tan Y-X, Chen Y-K, You H-J. Sex-related differences in descending norepinephrine and serotonin controls of spinal withdrawal reflex during intramuscular saline induced muscle nociception in rats. Exp Neurol. 2011;228:206–214. doi: 10.1016/j.expneurol.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Lopez R, Cox VC. Analgesia for tonic pain by self-administered lateral hypothalamic stimulation. Neuroreport. 1992;3:311–314. doi: 10.1097/00001756-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Lum J, Nguyen T, Felpel L. Drug distribution in solid tissue of the brain following chronic, local perfusion utilizing implanted osmotic minipumps. J Pharmcol Method. 1984;12:141–147. doi: 10.1016/0160-5402(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Martin J. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Decending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Monda M, Amaro S, Sullo A, De Luca B. Lateral hypothalamus lesion induces sympathetic stimulation and hyperthermia by activating synthesis of cerebral prostaglandins. Prostaglandins. 1996;51:169–178. doi: 10.1016/0090-6980(96)00001-9. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. Adipocyte. 2012;1:116–120. doi: 10.4161/adip.19736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD, Hoch DB. 14C-dopamind microinjected into the brain-stem of the rat: Dispersion kinetics, site content and functional dose. Brain Res Bull. 1978;3(6):601–609. doi: 10.1016/0361-9230(78)90006-0. [DOI] [PubMed] [Google Scholar]
- Mylius V, Kunz M, Schepelmann K, Lautenbacher S. Sex differences in nociceptive withdrawal reflex and pain perception. Somatosens Mot Res. 2005;22:207–211. doi: 10.1080/08990220500262414. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Reg-I. 2011;301:R1207–1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central afferent pathways mediating skin cooling-evoked sympathethic thermogenesis in brown adipose tissue. Am J Physiol Reg-I. 2007;292:R127–136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Niesters M, Aarts L, Sarton E, Dahan A. Influence of ketamine and morphine on descending pain modulation in chronic pain patients: a randomized p;acebo-controlled cross-over proof-of-concept study. Brit J Anaesth. 2013;110:1010–1016. doi: 10.1093/bja/aes578. [DOI] [PubMed] [Google Scholar]
- Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine anesthesia by site specific n-methyl-D-Aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin Psychiat. 2011;72:219–224. doi: 10.4088/JCP.08m04969blu. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rager G, Kretz R. ON and OFF regions in layer IV of striate cortex. Brain Res. 1985;327:319–323. doi: 10.1016/0006-8993(85)91527-6. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colville LM, McKinley MJ. The neurochemical characterization of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Olessen SS, Brock C, Krarup AL, Funch-Jensen P, Arendt-Nielsen L, Wilder-Smith OH, Drewes AM. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastronenterol H. 2010;8:724–730. doi: 10.1016/j.cgh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Reg-I. 2004;287:R306–313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- Osaka T. Heat loss responses and blockade of prostaglandin E2-induced thermogenesis elicited by alpha1-adrenergic activation in rostromedial preoptic area. Neuroscience. 2009;162:1420–1428. doi: 10.1016/j.neuroscience.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Osaka T. Thermoregulatory responses elicited by microinjection of L-glutamate and its interaction with thermogenic effects of GABA and prostaglandin E2 in the preoptic area. Neuroscience. 2012;226:156–164. doi: 10.1016/j.neuroscience.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watdon C. The Rat Brain in Stereotaxic Coordinates. Elsevier; Amsterdam: 2009. [Google Scholar]
- Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain. 2002;6(4):295–305. doi: 10.1053/eujp.2002.0339. [DOI] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimenta pain perception – Part 1: Are there really differences between women and men? Pain. 2012;153:602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Carlisle HJ. Effects of lateral hypothalamic lesions on thermoregulation in rat. Physiol Behav. 1986;38:219–228. doi: 10.1016/0031-9384(86)90157-5. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Carlisle HJ. A reevaluation of the role of the lateral hypothalamus in behavioral temperature regulation. Physiol Behav. 1987;40:189–192. doi: 10.1016/0031-9384(87)90206-x. [DOI] [PubMed] [Google Scholar]
- Richard D. Energy expenditure: A critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol. 2007;32:173–183. [PubMed] [Google Scholar]
- Rojo ML, Rodriguez-Gaztelumendi A, Pazos A, Diaz A. Differential adaptive changes on serotonin and noradrenaline transporters in a rat model of peripheral neuropathic pain. Neurosci Lett. 2012;515(2):181–186. doi: 10.1016/j.neulet.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Safari M-S, Haghparast A, Semnanian S. Effect of lidocaine administration at the nucleus locus coeruleus on lateral hypothalamus-induced antinociception in the rat. Pharmacol Biochem Behav. 2009;92(4):629–634. doi: 10.1016/j.pbb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Sakai M, Swartz B, Woody C. Controlled micro release of pharmacological agents: Measurements of volume ejected in vitro through fine tipped glass microelectrodes by pressure. Neuropharmacology. 1979;18:209–213. doi: 10.1016/0028-3908(79)90063-7. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Schiene K, De Vry J, Tzschentke TM. Anitnociceptive and antihypertensive effects of tapentadol in animal models of inflammatory pain. J Pharm Exp Ther. 2011;339:537–544. doi: 10.1124/jpet.111.181263. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav R. 2010;34:177–184. doi: 10.1016/j.neubiorev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schroder W, De Vry J, Tzschentke TM, Jahnel, Christoph T. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur J Pain. 2010;14(8):814–821. doi: 10.1016/j.ejpain.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, Maihöfner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain. 2009;132:788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Brit J Pharmacol. 165(7):2292–303. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Samson WK, Ferguson AV. Cardiovascular actions of orexin-A in the rat subfornical organ. J Neuroendocrinol. 2007;19:7–13. doi: 10.1111/j.1365-2826.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptorlike protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 2009;161:475–488. doi: 10.1016/j.neuroscience.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzedo-Rodrigues LS, Depieri T, Cherobino AJ, Lopes OU, Menani JV, Colombari DS. Hypothalamic disconnection caudal to paraventricular nucleus affects cardiovascular and drinking responses to central angiotensin II and carbachol. Brain Res. 2011;1388:100–108. doi: 10.1016/j.brainres.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–475. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- Wagner MA, Kuhnke S, Holden JE. International Association for the Study of Pain Abstracts. 2012. Differences in dose, sex, and pain type. [Google Scholar]
- Xiao F, Jiang M, Du D, Xia C, Wang J, Cao Y, Shen L, Zhu D. Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2012;67:16–24. doi: 10.1016/j.neuropharm.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Xu B, Descalzi G, Ye H-R, Zhuo M, Wang Y-W. Translational investigation and treatment of neuropathic pain. Mol Pain. 2012;8:15. doi: 10.1186/1744-8069-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in rat. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]