Abstract
Substantial behavioral evidence exists to support the idea that the lateral hypothalamus (LH) makes axonal connection with spinally-projecting noradrenergic neurons of the A7 catecholamine cell group in the pons. Through this putative projection, the LH modulates nociception via α1- and α2-adrenoceptors in the dorsal horn. We used double-label immunocytochemistry to demonstrate that axons from the LH labeled with the anterograde tracer biotinylated dextran amine (BDA) appose tyrosine hydroxylase-immunoreactive (TH-ir) neuron profiles in the A7 area. Other pontine areas labeled with BDA included the dorsomedial tegmental area, the pontine reticular nucleus, oral part, the caudal aspect of the dorsal raphe, the periaqueductal grey and the A6 area. To confirm the findings of the brightfield experiment, we used confocal microscopy to identify axons from the LH labeled with the anterograde tracer Fluoro-Ruby co-localized with TH-ir dendrites and cell bodies in the A7 cell group. These findings provide an anatomical substrate for behavioral studies in which stimulation of the LH modifies nociception in the spinal cord via norepinephrine.
It is well known that spinally-projecting noradrenergic neurons from the pontine A7 catecholamine cell group play a role in the descending modulation of pain [1,2,3,4]. Axons from A7 neurons innervate laminae I and II of the spinal cord dorsal horn, areas known to process nociceptive input from the periphery [5], and stimulation of the A7 cell group in rat produces antinociception mediated by α2-adrenoceptors, but pronociception mediated by α1-adrenoceptors in the dorsal horn [6,7,8]. The lateral hypothalamus (LH) is also recognized as part of the descending pain modulatory system [9,10,11,12,13,14]. Stimulation of the LH produces an opposing action of norepinephrine in the dorsal horn similar to that seen by direct stimulation of the A7 cell group; that is, antinociception mediated by α2-adrenoceptors, but pronociception mediated by α1-adrenoceptors in the dorsal horn, in both a model of inflammatory nociception [11] and a model of acute nociception [12]. Anatomical studies provide evidence of a direct LH to dorsal horn connection [15,16], but there is no evidence for spinally-projecting noradrenergic neurons in the LH. Taken together, the evidence is suggestive that the LH effects noradrenergic modulation in the dorsal horn through connections with discrete, spinally projecting monoamine cells in the brainstem, such as the A7 cell group.
To test this hypothesis, we used anterograde tract tracing combined with double label immunocytochemistry to demonstrate axonal connections from neurons in the LH with dendrites and cell bodies of tyrosine hydroxylase – immunoreactive (TH-ir) neurons in the A7 area.
EXPERIMENTAL PROCEDURES
This study was done at the University of Illinois at Chicago and the Institutional Animal Care Committee approved the experimental protocols. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and EC Directive 86/609/EEC. All efforts were made to minimize animal suffering, reduce the numbers of animals used, and use alternatives to in vivo experiments.
Animals
Female Sprague-Dawley rats (250–350 g, Charles River, Portage, MI, USA) were used. Rats were kept on a 12-hour day/night schedule with free access to food and water.
Tracer Iontophoresis
Each of twelve rats was deeply anesthetized with sodium pentobarbital (50 mg/kg) and surgically prepared using aseptic technique. A glass micropipette with a tip diameter of 35–40 um was filled with either a 10% solution of BDA (Molecular Probes, Eugene, OR, USA; n = 6) or Fluoro-Ruby (Fluorochrome, Inc., Denver, CO, USA; n = 6) and lowered into the LH through a burr hole to a point defined by the following stereotaxic coordinates: -1.5 mm from bregma, lateral +1.7 mm, vertical +2.2 mm, incisor bar set at +2.5mm. BDA or Fluoro-Ruby was iontophoretically deposited using 5–15 μA positive current pulses of 500ms duration and delivered at a rate of 0.5Hz for 20 min. The pipette was kept in place for an additional 60s before removal to minimize diffusion of tracer into the pipette track. Two weeks later, each rat was deeply anesthetized with pentobarbital and the brains fixed by transcardial perfusion with the following solutions: 100 ml of normal saline followed by 200 ml of cold 4% paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4), and 100 ml 10% sucrose in 0.1M PB.
Tissue processing
Forebrain tissue blocks containing the LH and brainstem blocks containing the dorsolateral pontine tegmentum were frozen, 40-μm transverse sections were cut on a cryostat microtome, and free-floating sections were processed for visualization of BDA and tyrosine hydroxylase-immunoreactivity as previously described [17,18]. Briefly, sections were first processed to visualize BDA using the avidin – biotin complex (Elite Vectastain ABC, Vector Laboratories, Inc., Burlingame, CA, USA) and the nickel-enhanced 3,3'-Diaminobenzidine (DAB) chromogen reaction. Following rinses, sections were incubated for 60–90 min in a solution containing the avidin–biotin complex. The blue–black nickel-enhanced DAB chromogen was developed by incubating sections in a solution containing 0.05 M Tris–HCl (pH 7.6), 0.67% nickel ammonium sulfate, filtered 0.013% DAB and 0.005% hydrogen peroxide. Sections from the pons were incubated overnight in a solution containing mouse antisera directed against tyrosine hydroxylase (TH; IncStar Co., Stillwater, MN, USA) diluted 1:2000 with phosphate buffered saline (PBS) and 0.1% Triton X-100. Following rinses, sections were incubated for 60 min in a solution containing rabbit anti-mouse secondary antibody (Jackson ImmunoResearch Inc., West Grove, PA, USA) diluted 1:80 with PBS and then rinsed. Sections were then incubated for 60 min in a solution containing mouse PAP complex (Cappel Laboratories Inc., Cochranville, PA, USA) diluted 1:150 with PBS, and rinsed. The brown DAB chromogen was developed by incubating the sections for 6 min in a solution that contained 0.05% filtered DAB and 0.005% hydrogen peroxide in Tris–HCl buffer at pH 7.6.
Fluoro-Ruby brains were perfused as previously described, removed and placed in a 4% paraformaldehyde solution for 4h, and then transferred to a 20% sucrose solution for 48 h. Tissue blocks containing the LH and the A7 cell group were then sliced as described. Sections containing the A7 area were incubated in blocking solution (75 ml PBS, 0.6 gram of 0.8% bovine albumin, Sigma, St. Louis, MO, USA); 0.74 ml of 0.2% Amersham gelatin (GE Healthcare Life Sciences, Piscataway NJ, USA) and then immediately incubated for 48 h in primary antiserum made in mouse against TH diluted 1:2000 with PBS and containing 0.5% Triton X-100. After rinses, the tissues were incubated for 60 min in a secondary antibody made against rabbit and labeled with cyanine2 (Cy2; 1:200; Invitrogen, Austin, TX, USA) in 0.5% Triton X-100 in PBS. All sections were mounted on gel-coated slides, processed through alcohols and xylenes, and coverslipped.
Microscopy
BDA photomicrographs were made using a Zeiss Ax10 Imager M1. The low powered pontine section was photographed on Nikon E800 light microscope, as was the Fluoro-Ruby injection site, but with a fluorescence TRITC filter. Sections containing the A7 cell group were photographed using a Zeiss LSM 510 META confocal microscope. Stack images were taken at 100X magnification. No image corrections were made for contrast or color.
RESULTS
This is a descriptive study in which anterograde transport of BDA or Fluoro-Ruby was used to identify the locations of axons in apposition to, and co-localized with, TH-ir soma and dendrites in the A7 area. Both of the injection sites for BDA and Fluoro-Ruby were located in the tuberal aspect of the LH, between the fornix and the internal capsule. Figure 1 shows a core of compacted tracer for BDA (A) and Fluoro-Ruby (B) in the LH with some labeling of neuron cell bodies and processes in the area surrounding the compaction.
Figure 1.
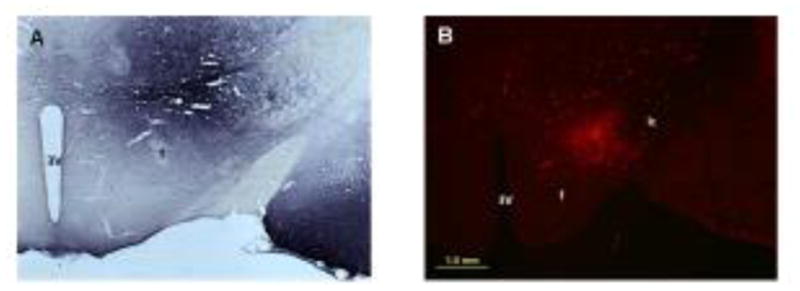
Photomicrographs (2x) of BDA (A) and Fluoro-Ruby (B) injections in the LH that resulted in anterograde labeling of axons seen in Figs. 2–5. The core of compacted tracer at the center of the injection can be seen in both photos. A halo of neurons around the core represent local labeling of neurons. Abbreviations: 3V, third ventricle; f, fornix, ic, internal capsule. Scale bar = 1 mm. Distance from bregma is approximately −3.36 mm.
Brightfield Microscopy
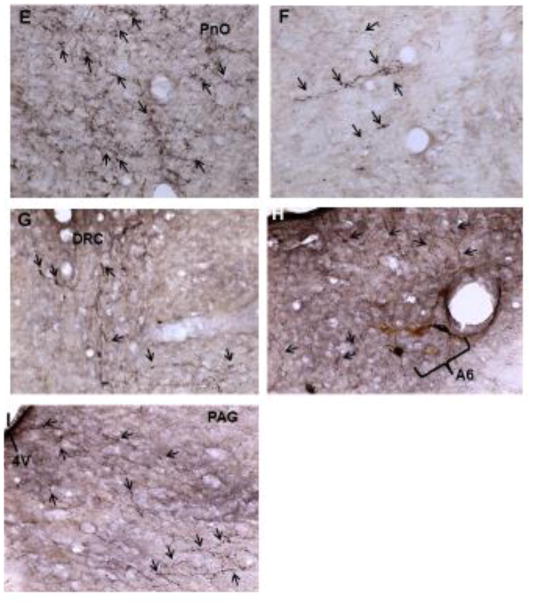
Generally, the most densely labeled axons were seen in the ipsilateral dorsomedial tegmentum medial to the A7 area (Figure 2C) and in the pontine reticular nucleus oralis (Figure 2E). This labeling continued through the rostro-caudal extent of the pons, although the labeling was much less in the caudal aspect of the pons. Contralateral projections were much more limited (Figures 2D and F). Also noted were a number of BDA labeled axons in the central aspect of the caudal dorsal raphe nucleus (Figure 2G) and in apposition to TH-ir neuron profiles in the A6 area (Figure 2H). BDA labeled axons were also seen in the ventrolateral periaqueductal gray (Figure 2I). In the dorsal raphe, two large multipolar neuron profiles were labeled purple-black, and in the A7 area, a few neuron profiles were lightly labeled with BDA-ir.
Figure 2.
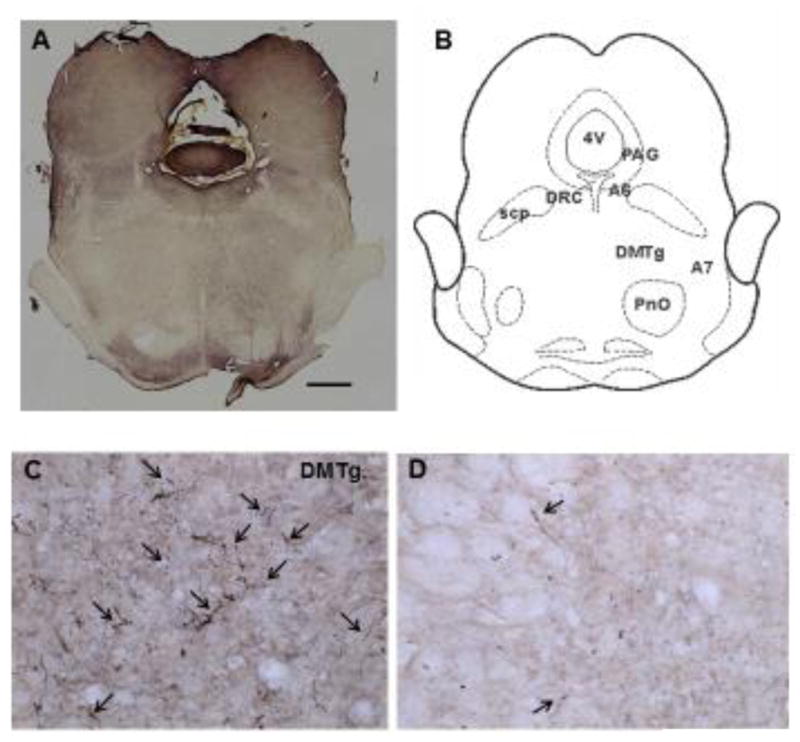
(A) A low powered section from the pons (4x) at −8.64 bregma (scale bar = 1000μ). Compare to the cartoon of the section (B). Higher powered photomicrographs of axons anterogradely labeled with BDA in the pons (20x) were taken from this section (C–I). Moderately dense labeling can be seen in the ipsilateral dorsomedial tegmentum (C), with numerous varicosities in evidence (arrows). Only a few BDA-labeled axons were observed on the contralateral side (D). Similarly, a dense field of labeled axons was seen in the ipsilateral pontine reticular nucleus oralis adjacent ventrally to the dorsomedial tegmentum (E), while only a few scattered labeled axons were noted in the contralateral side (F). Moderately dense axon labeling was found near the midline caudal dorsal raphe nucleus (G). Scattered axons were also seen near A6 catecholaminergic neurons ipsilateral to the BDA injection (H) and in the ventrolateral PAG (I). Abbreviations: 4V, 4th ventricle; A6, A6 catecholamine cell group; A7, A7 catecholamine cell group; DMTg, dorsomedial tegmental area; DRC, dorsal raphe nucleus, caudal part; PAG, periaqueductal gray; PnO, pontine reticular nucleus, oral part; scp, superior cerebellar peduncle.
In the A7 area, TH-ir neuron profiles were found in the dorsolateral pontine tegmentum below the superior cerebellar peduncle and adjacent to the Kölliker-Füse nucleus. These profiles were multipolar, approximately 30–40 μ in diameter, and with some dendritic processes visible. BDA labeled axons were primarily ipsilateral to the LH injection site. Varicosities were clearly seen on a number of the labeled axons. Figures 3A and 3B are 40x magnifications of TH-ir neuron profiles in the A7 area with BDA-labeled axons in the same plane of focus.
Figure 3.
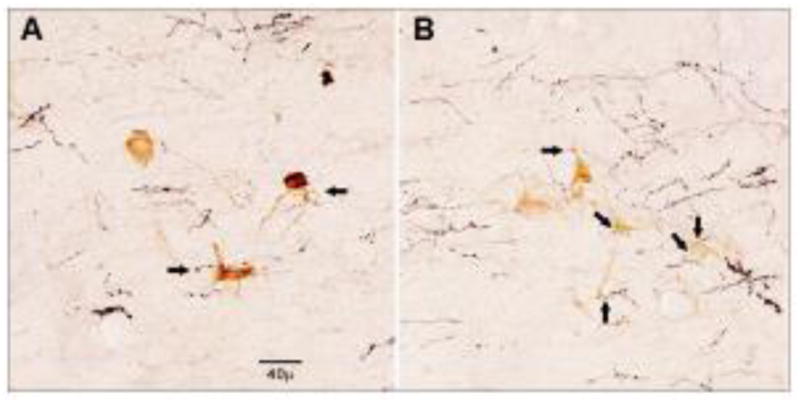
High powered (40X) magnification of neuron profiles in the A7 area immunoreactive for TH in the same plane of view as axons labeled with BDA. Arrows indicate putative synaptic connections for both dendrites and cell bodies. Scale bar = 40μ. This photomicrograph is from the A7 area in Figure 2A.
Co-localization of TH-ir profiles with Fluoro-Ruby-containing axons
To confirm the findings of the BDA study, we used confocal microscopy with Fluoro-Ruby as the anterograde tracer and Cy2 as the label for TH-ir. Figures 4 and 5 show green TH-ir neuron profiles in the A7 area with red axons labeled with Fluoro-Ruby from the injection in the LH. Co-localization can be seen in the two different brains as indicated by yellow puncta. In Figure 4, Fluoro-Ruby-labeled axons co-localize with TH-ir dendrites (A–D). Figure 5 shows Fluoro-Ruby labeled axons co-localizing with both TH-ir cell bodies and dendrites (A–C).
Figure 4.
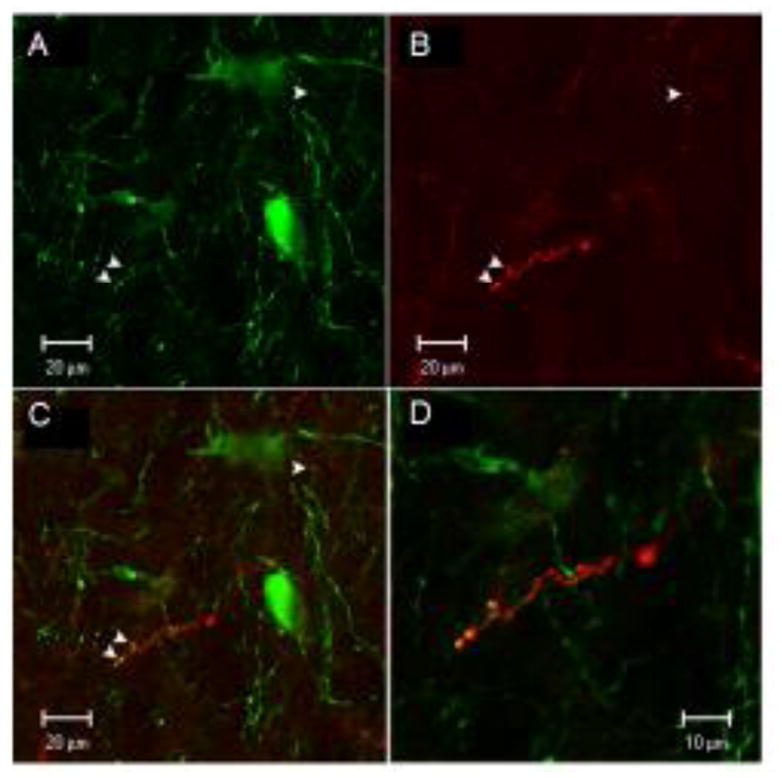
Confocal images showing neuron profiles labeled with green TH-ir in the A7 cell group (A), axons labeled with Fluoro-Ruby from the injection site in 1B (B), and co-localization of Fluoro-Ruby labeled axons with TH-ir dendrites (C). Arrows show yellow puncta at sites of co-localization. Panel D is an enlargement of panel C.
Figure 5.
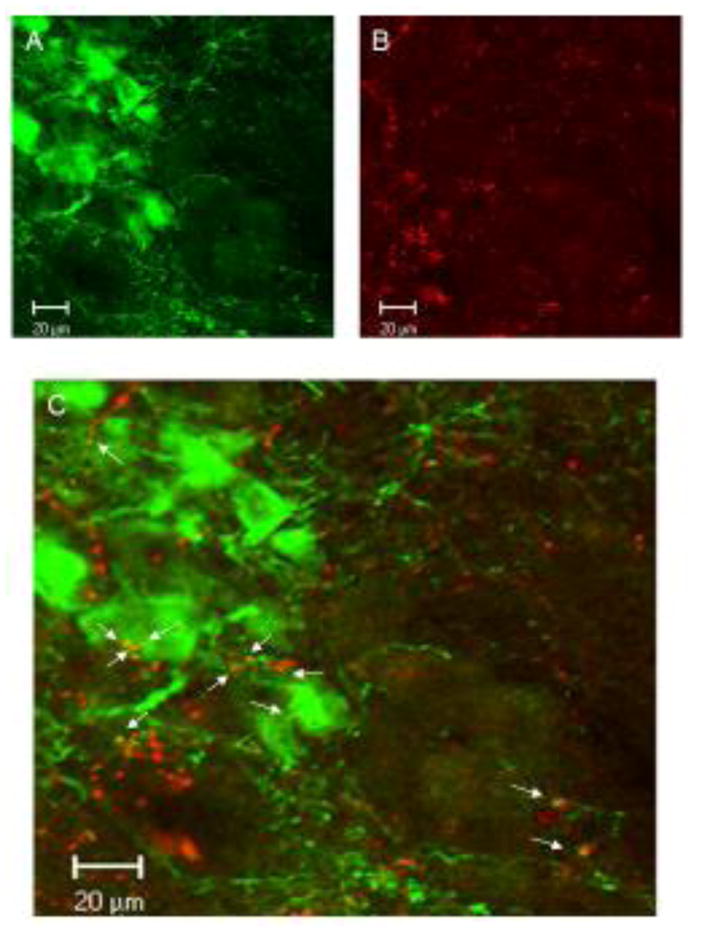
Confocal images of TH-ir neuron profiles in the A7 area (A) with Fluoro-Ruby labeled axons form an injection in the LH similar to that shown in 1B (B), with co-localization of the axons with TH-ir dendrites and cell bodies, as shown by yellow puncta (arrows; C).
DISCUSSION
The findings from the present study provide anatomical evidence to support behavioral studies indicating that neurons in the LH project in part to spinally-descending neurons in the A7 catecholamine group. Through this connection, the LH modifies nociception at the spinal cord dorsal horn in part via norepinephrine acting on α1- and α2-adrenoceptors.
Our study was in no way meant to be a comprehensive study of LH to pons neuronal connections. We attempted to place the anterograde tracers in the LH in the approximate area that we make microinjections of carbachol in our behavioral studies, but the present findings do not provide conclusive evidence that the same neurons stimulated by carbachol microinjection in the LH were the same neurons labeled by BDA or Fluoro-Ruby. However, the findings of the present study combined with behavioral reports provide converging evidence of an anatomical substrate for LH behavioral studies [11,12].
We did not examine the neurochemical content of anterogradely labeled neurons for this small study. Two likely neurotransmitter candidates for LH to A7 projections are substance P and orexins. Substance P-ir neurons are found in the LH and a moderate amount of substance P-ir axons have been identified in the dorsolateral pontine tegmentum [19,20], some of which make synaptic contacts with TH-ir dendrites in the A7 area [21]. Noradrenergic neurons in the A7 area increase their firing rates when exposed to substance P [21], microinjection of substance P in the A7 area produces antinociception [6], and microinjection of the neurokinin1 receptor antagonist, L-703,606, into the A7 area reduces LH-induced antinociception [13]. Orexins-containing neurons are prevalent in the LH and are known to play a role in nociceptive modulation either by a direct connection to the spinal cord dorsal horn or at supraspinal levels [22,23,24]. LH orexin-containing neurons project to the parabrachial area [25], but no behavioral studies have been done demonstrating an LH to A7 orexins connection. Other potential neurotransmitter candidates include met-enkephalin [26,27] and glutamate [28].
This study focused on the actions of LH neurons only in the noradrenergic descending modulatory system. As mentioned earlier, LH stimulation also activates orexin–containing neurons in the LH that project directly to the dorsal horn [24,25], and possibly met-enkephalin [27] and glutamate neurons [28]. Similarly, LH innervation of the A7 area may activate non- noradrenergic neurons, including GABA and glycine neurons that are known to exist near the A7 cells [7,21]. It is likely that the overall antinociceptive effect of LH stimulation is a net effect, not just of opposing α1- and α2-adrenoceptors, but of multiple neurotransmitters that exhibit excitatory and inhibitory effects on various neurons in the dorsal horn. While this complexity makes study of the descending system difficult, it also provides a wealth of targets for the development of therapeutics.
The current study only examined unilateral, left-sided LH to A7 projections in female rats. Sex differences exist in the structural networks of the brain such that males exhibit greater facilitation in perception and coordinated movements, and female brains are structured to facilitate the connection between analytical and intuitive processing of information [29]. Behavioral responses are suggestive of a sex difference in descending pain modulation from the LH to the A7. Antinociception in male rats is blocked when cobalt chloride is microinjected in the left LH, while blocking synaptic activity in the left LH of female rats does not reverse antinociception [14]. There are also sex differences when different doses of carbachol are used to stimulate the LH [30]. It is possible that a sex difference in the LH to A7 projection could be responsible in part for these sex differences in behavioral responses. This notion is supported by documented sex differences in pain modulation through the ventrolateral periaqueductal gray [31], as well as differences in microglial activity during allodynia development [32]. In order to fully understand the connection between the LH and A7, further research exploring the right-sided projection and the LH to A7 connection in male rats is needed.
In the current study, small diameter glass pipettes were used to limit the spread of tracer and to reduce damage to fibers of passage, and only those injections within the LH were analyzed [18]. We did observe two neurons in the A6 area that were labeled with BDA, and several neuron profiles very lightly labeled with BDA in the A7 area. These small numbers of retrogradely labeled neuron profiles likely did not contribute substantially to the BDA labeled axons in the A7 area, but a complete study utilizing newer anterograde tracers that rarely transport retrogradely is needed [33].
In conclusion, this study provides an anatomical substrate to support behavioral evidence that the LH modifies nociception in the spinal cord dorsal horn in part through connections with spinally projecting noradrenergic neurons in the A7 catecholamine cell group.
Highlights.
Axons from neurons in the lateral hypothalamus apposed neurons in the A7area.
Axons from neurons in the lateral hypothalamus co-localized in the A7 cell group.
Results supported behavioral studies for lateral hypothalamic modulation of pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nam H, Kerman IA. Distribution of catecholaminergic presympathetic-premotor neurons in the rat lower brainstem. Neuroscience. 2016 Jun 2;324:430–445. doi: 10.1016/j.neuroscience.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013 Sep;716(1–3):2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 3.Mravec B, Bodnar I, Uhereczky G, Kvetnansky R, Palkovits M. Effect of lesions of A5 or A7 noradrenergic cell group or surgical transection of brainstem catecholamine pathways on plasma catecholamine levels in rats injected subcutaneously by formalin. Gen Physiol Biophys. 2012 Sep;31(3):247–254. doi: 10.4149/gpb_2012_029. [DOI] [PubMed] [Google Scholar]
- 4.Bajic D, Commons KG. Visualizing acute pain-morphine interaction in descending monoamine nuclei with Fos. Brain Res. 2010 Jan 8;1306:29–38. doi: 10.1016/j.brainres.2009.10.010. Epub 2009 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;547(2):279–288. doi: 10.1016/0006-8993(91)90972-x. [DOI] [PubMed] [Google Scholar]
- 6.Yeomans DC, Proudfit HK. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience. 1992;49(3):681–691. doi: 10.1016/0306-4522(92)90236-u. [DOI] [PubMed] [Google Scholar]
- 7.Nuseir K, Proudfit HK. Bidirectional modulation of nociception by GABA neurons in the dorsolateral pontine tegmentum that tonically inhibit spinally projecting noradrenergic A7 neurons. Neuroscience. 2000;96(4):773–783. doi: 10.1016/s0306-4522(99)00603-x. [DOI] [PubMed] [Google Scholar]
- 8.Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by alpha1- and alpha2-adrenoceptors. Neuroscience. 1999;91(3):979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- 9.Aimone LD, Gebhart GF. Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Res. 1987;403:290–300. doi: 10.1016/0006-8993(87)90066-7. [DOI] [PubMed] [Google Scholar]
- 10.Franco AC, Prado WA. Antinociceptive effects of stimulation of discrete sites in the rat hypothalamus: evidence for the participation of the lateral hypothalamus area in descending pain suppression mechanisms. Braz J Med Biol Res. 1996;29:1531–1541. [PubMed] [Google Scholar]
- 11.Jeong Y, Holden JE. Lateral hypothalamus-induced alpha-adrenoceptor mediated nociceptive modulation acts on c-fibers in rat. Biological Research for Nursing. 2009;10(4):331–339. doi: 10.1177/1099800408325053. [DOI] [PubMed] [Google Scholar]
- 12.Holden JE, Naleway E. Microinjection of carbachol in the lateral hypothalamus produces opposing actions on nociception mediated by alpha(1)- and alpha(2)-adrenoceptors. Brain Res. 2001;911:27–36. doi: 10.1016/s0006-8993(01)02567-7. [DOI] [PubMed] [Google Scholar]
- 13.Holden JE, Van Poppel AY, Thomas S. Antinociception from lateral hypothalamic stimulation is mediated by NK1 receptors in the A7 catecholamine cell group in the rat. Brain Research. 2002;953:195–204. doi: 10.1016/s0006-8993(02)03285-7. [DOI] [PubMed] [Google Scholar]
- 14.Wagner M, Banerjee T, Jeong Y, Holden JE. Sex differences in hypothalamic-mediated tonic norepinephrine release for thermal hyperalgesia in rats. Neuroscience. 2016;324:420–429. doi: 10.1016/j.neuroscience.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in rat. J Comp Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 16.Jones SL. Descending control of nociception. In: Light AH, editor. Pain and Headache, The Initial Processing of Pain and Its Descending Control: Spinal and Trigeminal Systems. Karger; Basil: 1992. pp. 201–295. [Google Scholar]
- 17.Holden JE, Proudfit HK. Enkephalin neurons that project to the A7 catecholamine group are located in nuclei that modulate nociception: Ventromedial medulla. Neuroscience. 1998;83:929–947. doi: 10.1016/s0306-4522(97)00437-5. [DOI] [PubMed] [Google Scholar]
- 18.Bajic D, Proudfit HK. Projections from the rat cuneiform nucleus to the A7, A6 (locus coeruleus), and A5 pontine noradrenergic cell groups. J Chem Neuroanat. 2013 May;50–51:11–20. doi: 10.1016/j.jchemneu.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat—I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- 20.Proudfit HK, Monsen M. Ultrastructural evidence that substance P neurons form synapses with noradrenergic neurons in the A7 catecholamine cell group that modulate nociception. Neuroscience. 1999;91(4):1499–1513. doi: 10.1016/s0306-4522(98)00716-7. [DOI] [PubMed] [Google Scholar]
- 21.Min M-Y, Wu Y-W, Shih P-Y, Lu H-W, Lin C-C, Wu Y, Li M-J, Yang H-W. Physiological and morphological properties of, and effect of substance P on, neurons in the A7 catecholamine cell group in rats. Neuroscience. 2008;153:1020–1033. doi: 10.1016/j.neuroscience.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Chiou LC, Lee HJ, Ho YC, Chen SP, Liao YY, Ma CH, Fan PC, Fuh JL, Wang SJ. Orexins/hypocretins: pain regulation and cellular actions. Curr Pharm Des. 2010;16(28):3089–100. doi: 10.2174/138161210793292483. [DOI] [PubMed] [Google Scholar]
- 23.Jahangirvand M, Yazdi F, Moradi M, Haghparast A. Intra-accumbal orexin-1 receptors are involved in antinociception induced by stimulation of the lateral hypothalamus in the formalin test as an animal model of persistent inflammatory pain. Iran J Pharm Res. 2016 Fall;15(4):851–859. [PMC free article] [PubMed] [Google Scholar]
- 24.Wardach J, Wagner M, Jeong Y, Holden JE. Lateral hypothalamic stimulation reduces thermal hyperalgesia through spinally descending orexin-A neurons in neuropathic pain. Western Journal of Nursing Research. 2016;38:292–307. doi: 10.1177/0193945915610083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pego-Reigosa R, Coveñas R, Tramu G, Pesini P. Distribution of met-enkephalin immunoreactivity in the diencephalon and the brainstem of the dog. J Chem Neuroanat. 2000 Sep;19(4):243–258. doi: 10.1016/s0891-0618(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez ML, Díaz-Cabiale Z, Narváez JA, Manso B, Salinas P, Rivada EV, Coveñas R. Mapping of methionine-enkephalin-arg6-gly7-leu8 in the human diencephalon. Neuroscience. 2016;334:245–258. doi: 10.1016/j.neuroscience.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Poller WC, Madai VI, Bernard R, Laube G, Veh RW. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013 Apr 24;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014 Jan 14;111(2):823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holden JE, Wang E, Moes JR, Wagner M, Maduko A, Jeong Y. Differences in carbachol dose, pain condition, and sex following lateral hypothalamic stimulation. Neuroscience. 2014 Jun 13;270:226–35. doi: 10.1016/j.neuroscience.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonsfeldt KJ, Suchland KL, Beeson KA, Lowe JD, Li MH, Ingram SL. Sex differences in GABAA signaling in the periaqueductal gray induced by persistent inflammation. J Neurosci. 2016 Feb 3;36(5):1669–81. doi: 10.1523/JNEUROSCI.1928-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011 Oct 26;31(43):15450–4. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vujovic N, Gooley JJ, Jhou TC, Saper CB. Projections from the subparaventricular zone define four channels of output from the circadian timing system. Journal of Comparative Neurology. 2015;523:2714–2737. doi: 10.1002/cne.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]