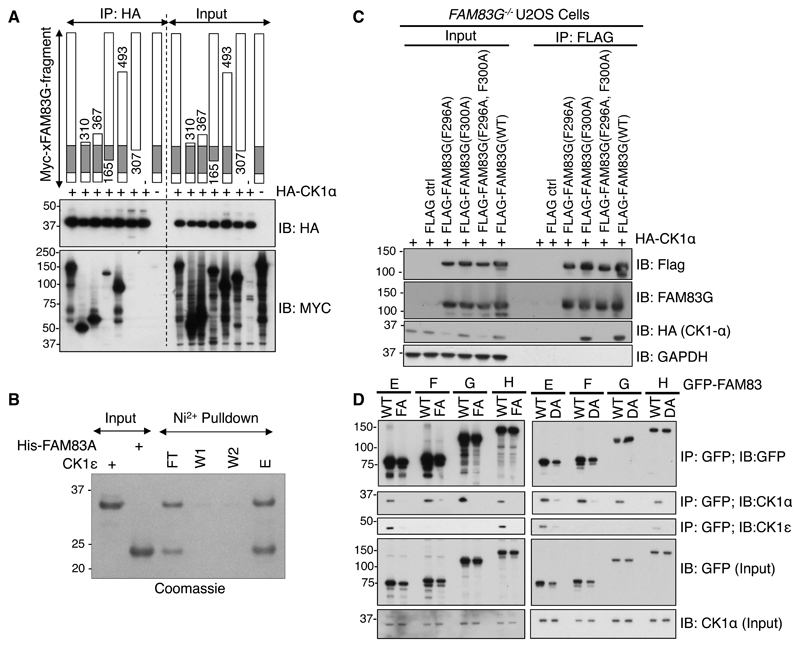
Fig. 3. The DUF1669 domain is sufficient to mediate the interaction of FAM83 proteins with CK1.
A. The indicated fragments of Myc-tagged Xenopus laevis FAM83G (Myc-xFAM83G) were co-expressed with HA-CK1α in FAM83G-/- U2OS cells, and then cell extracts or HA immunoprecipitates were subjected to immunoblotting (IB) with antibodies recognizing Myc or HA as indicated. This blot is representative of 3 independent experiments. B. A His-tagged fragment of FAM83A (amino acids122-304), which contains the DUF1669 and PLD-like domains, was mixed with recombinant CK1ε kinase domain (amino acids 1-294) in vitro. His-FAM83A(122-304) was then pulled down using Ni-sepharose (Ni2+) resin, which was washed twice before elution. The input, unbound flow-through (FT), wash solutions (W1 and W2), and eluate (E) were analysed by SDS-PAGE and stained with Coomassie blue. This gel is representative of 3 independent experiments. C. Empty Flag vector (ctrl) or the indicated FLAG-FAM83G mutant and wild-type (WT) proteins were overexpressed in FAM83G-/- U2OS cells. Cell extracts (input) and FLAG immunoprecipitates (IP) were subjected to immunoblotting for FLAG, CK1α, or GAPDH as indicated. This blot is representative of 3 independent experiments. D. WT and Phe→Ala (FA) and Asp→Ala (DA) mutant forms of GFP-FAM83E–H were transiently expressed in U2OS cells, immunoprecipitated (IP) from cell extracts with a GFP-specific antibody, and immunoblotted for GFP, CK1α, and CK1ε as indicated. This blot is representative of 3 independent experiments.