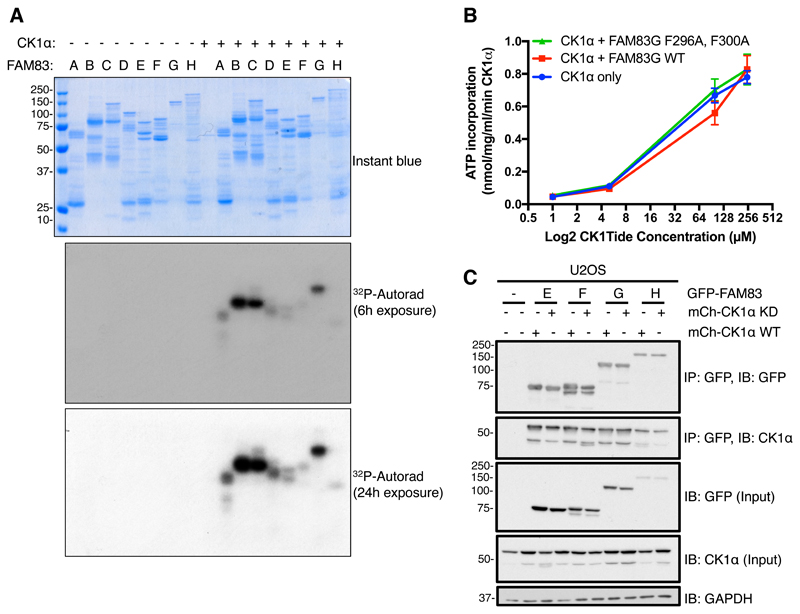
Fig. 9. The intrinsic catalytic activity of CK1 is not affected by or required for the association of CK1 with FAM83 proteins.
A. An in vitro kinase assay was performed in the presence of [γ32P]-ATP with recombinant GST-CK1α plus one of the following recombinant FAM83 fusion proteins: GST-FAM83A (A), MBP-FAM83B (B), GST-FAM83C (C), GST-FAM83D (D), GST-FAM83E (E), GST-FAM83F (F), GST-FAM83G-6His (G), or GST-FAM83H (H). After the reactions were stopped, samples were resolved by SDS-PAGE. The gel was stained with Instant blue, dried, and subjected to 32P autoradiography for the indicated times. Instant blue–stained gel and autoradiograph representative of 3 independent experiments are shown. B. An in vitro kinase assay was set up with recombinant GST-CK1α, and either recombinant GST-FAM83G-6His or the GST-FAM83G (F296A, F300A) double mutant in the presence of increasing amounts of the optimized CK1 peptide substrate CK1tide. GST-CK1α, without FAM83G addition, was used as a control. Data points represent the average from three independent experiments, each including three replicates. Error bars, SEM. C. U2OS cells were transiently co-transfected with GFP-FAM83E, GFP-FAM83F, GFP-FAM83G, or GFP-FAM83H and either WT CK1α or a catalytically inactive (kinase dead, KD) form of CK1α. After 24 h cell extracts (Input) were immunoprecipitated (IP) with GFP-TRAP A beads and immunoblotted (IB) with the indicated antibodies. This blot is representative of 3 independent experiments. GAPDH is a loading control.