Abstract
Scaling factors are reported for use in predicting 19F NMR chemical shifts for fluorinated (hetero)aromatic compounds with relatively low levels of theory. Our recommended scaling factors were developed using a curated data set of >50 compounds, with 100 individual 19F shifts spanning a range of >150 ppm. With a maximum deviation of 6.5 ppm between experimental and computed shifts, or 4% of the range tested, these scaling factors allow for the assignment of chemical shifts to specific fluorines in multifluorinated aromatics. The utility of this approach is highlighted by several structural reassignments.
Graphical Abstract
Introduction
The incorporation of fluorine atoms in organic molecules (e.g., materials,1 agrochemicals,2–3 pharmaceuticals4) is becoming increasingly common, as a result of the unique electronic and steric properties of fluorine atoms.5–12 Consequently, 19F NMR is applied ever more frequently in structural assignments. Fortunately for chemists working with fluorine, there are many appeals of using 19F NMR, e.g., the NMR active isotope is 100% naturally abundant, has a gyromagnetic ratio of about 0.94 times 1H, and is spin ½.13 Nonetheless, 19F spectra can be difficult to interpret, especially when multiple fluorine atoms are present in a molecule. Some of us have been involved in developing methods for regioselective defluorination of polyfluorinated aromatics, methods that produce exactly the sort of compounds subject to assignment difficulties.5–9, 14 Useful resources are available to assist organic chemists in the interpretation of 19F NMR, but it remains challenging to reliably assign all 19F shifts within a multifluorinated molecule.13 Here we endeavor to provide a readily accessible method for reliably predicting 19F shifts of fluorinated aromatic compounds.
Predicting 1H and 13C NMR chemical shifts with quantum chemical methods (especially density functional theory [DFT]) is now commonplace.15–18 Often, linear scaling methods are used with such approaches to remove systematic errors in computations.19–20 In doing so, absolute isotropic shieldings are computed for a “training set” of molecules and compared to experimental chemical shifts. When tight linear correlations between computed and experimental data are found, simple scaling factors (slope and y-intercept for the correlation line, particular to the specific theoretical method used) fall out, and can then be used for predicting chemical shifts for molecules not included in the training set. This approach has been explored previously for 19F chemical shifts, including fluorinated aromatic compounds,21–27,28 but we provide scaling factors here that work with relatively low level theoretical methods accessible to non-experts. We also demonstrate the applicability of these scaling factors for heterocycles (common in bioactive compounds) and highlight their use in assigning structures of multifluorinated compounds.
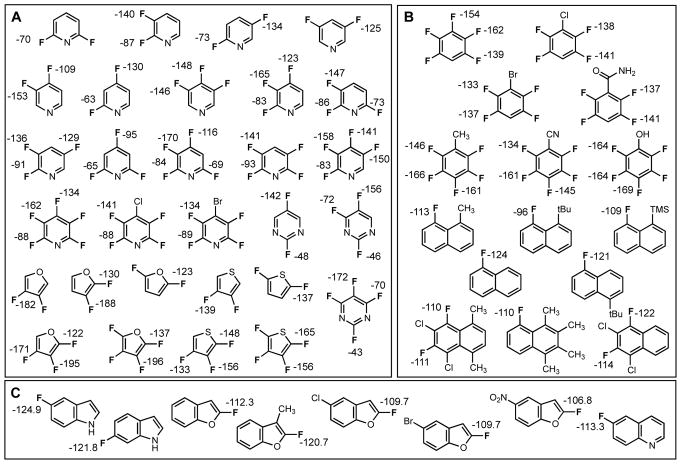
Our training set consisted of approximately 50 compounds, with 100 individual 19F shifts spanning a range of >150 ppm (Chart 1). This set contains fluorinated aromatics, many with multiple fluorine atoms in the same molecule, and mono- and bicyclic heteroaromatics. A variety of issues made compiling this data set difficult, including variations in reported shifts across the literature and the use of different reference compounds.27 The compounds in Chart 1 were chosen not just for the variety of environments in which their fluorine atoms reside, but because their 19F shifts were supported by multiple published reports or a single report with sufficient experimental details to make us confident in their validity.27, 29–42
Chart 1.
Experimental shifts of compounds used as training set to develop scaling factors: A: substituted mono-cyclic heterocylces, B: substituted non-heterocyclic aromatics, C: substituted bicyclic heterocycles.
Methods
Geometry optimizations were run using B3LYP/6-31+G(d,p) in the gas phase using Gaussian09, and resulting structures were confirmed as minima using frequency calculations.43–47,26 These structures were subjected to NMR (GIAO)48–54 calculations at B3LYP/6-31+G(d,p), B3LYP/6-311+G(2d,p) and B3LYP/6-31G levels in the gas phase. B3LYP/6-31+G(d,p)//B3LYP/6-31+G(d,p) calculations were repeated using the SMD implicit solvent model for experimental solvents in NMR calculations.55 Experimental shifts were plotted against calculated isotropic shielding values, and scaling factors were derived according to equation (1):
| (1) |
These scaling factors were applied to all each test compound using equation (2):
| (2) |
While the compounds in Chart 1 were chosen, in part, for their lack of conformational flexibility, some compounds to which the developed scaling factors were applied were conformationally flexible. For such compounds, conformational searches were run with Spartan10, and then optimized in Gaussian09 with B3LYP/6-31+G(d,p) in the gas phase. 26,56–57 Conformers within 3 kcal/mol of the lowest energy conformer were included in the NMR calculations, with their contributions weighted with a Boltzmann distribution based on their relative free energies.
Results and Discussion
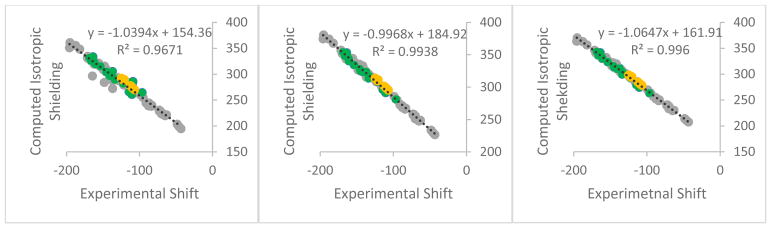
Using B3LYP/6-31+G(d,p)//B3LYP/6-31+G(d,p), the maximum error on any one 19F shift was 6.5 ppm, with a mean absolute deviation (MAD) of 2.1 ppm over a range of 153 ppm (Figure 1, middle). Performance was similar when NMR calculations were repeated with a larger basis set (B3LYP//6-311+G(2d,p))//B3LYP/6-31+G(d,p)): maximum error of 6.6 ppm and MAD of 1.7 ppm (Figure 1, right). Use of a smaller basis set (B3LYP/6-31G//B3LYP/6-31+G(d,p)) resulted in a much higher maximum deviation (28 ppm) and a much higher MAD of 4.0 ppm (Figure 1, left). On the basis of these results, we recommend the B3LYP/6-31+G(d,p)//B3LYP/6-31+G(d,p) for rapid predictions of 19F chemical shifts.
Figure 1.
Correlation data for two NMR computational methods (all using B3LYP/6-31+G(d,p) geometries): Left: B3LYP/6-31G Middle: B3LYP/6-31+G(d,p); Right: B3LYP/6-311+G(2d,p). Mono-cyclic aromatic compounds (group A, Chart 1) are shown in green, non-heterocyclic compounds (group B) are shown in grey, and bicyclic-heterocyclic compounds (group C) are shown in yellow.
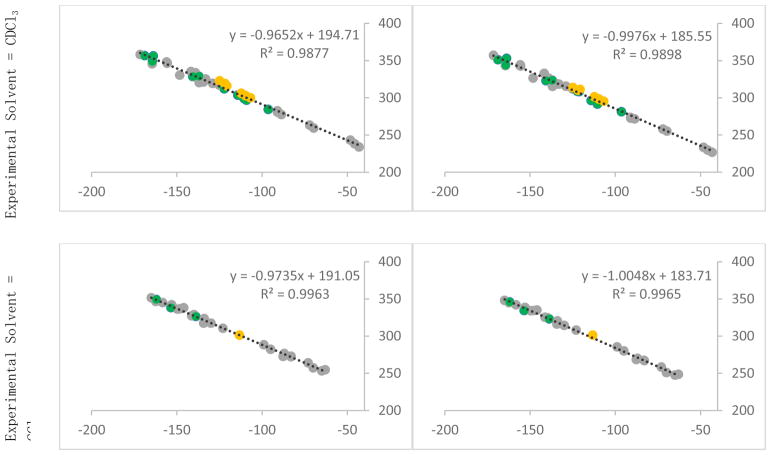
Although the 19F spectra for compounds used in the training set were taken in a variety of solvents, the majority were taken in either CDCl3 or CCl4 (a full list of the solvents is available in the Supporting Information). To test the effect of solvent on our predictions, the gas phase optimized structures were subjected to NMR calculations in continuum solvent (SMD) and new scaling factors were determined (Figure 2). For the 45 19F shifts from our test set that were measured in chloroform, gas phase NMR calculations had a maximum error of 8.2 ppm, while continuum chloroform calculations had a maximum error of 7.2 ppm. The MAD also decreased slightly, from 2.9 to 2.6 ppm. For the 27 shifts determined in carbon tetrachloride, the maximum error decreased from 5.1 to 4.6 ppm, while the MAD remained the same at 1.5 ppm. Although including solvent in the calculations did provide small improvements in prediction accuracy, gas phase calculations appear to be sufficient.
Figure 2.
Experimental and computed shifts for the training set used for optomization and NMR calculation at B3LYP/6-31+G(d,p) – structures taken in chloroform (top) and calculated in gas (left) and chloroform (right), structures taken in carbon tetrachloride (bottom). Mono-cyclic aromatic compounds (group A) are shown in green, non-heterocyclic compounds (group B) are shown in grey, and bicyclic-heterocyclic compounds (group C) are shown in yellow.
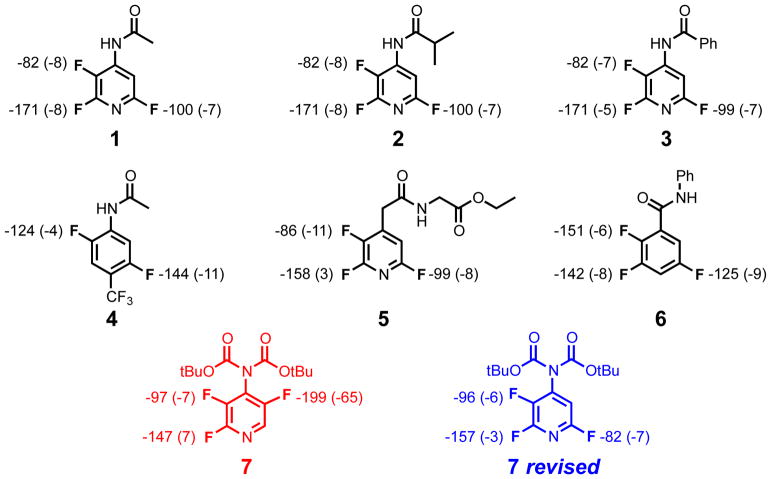
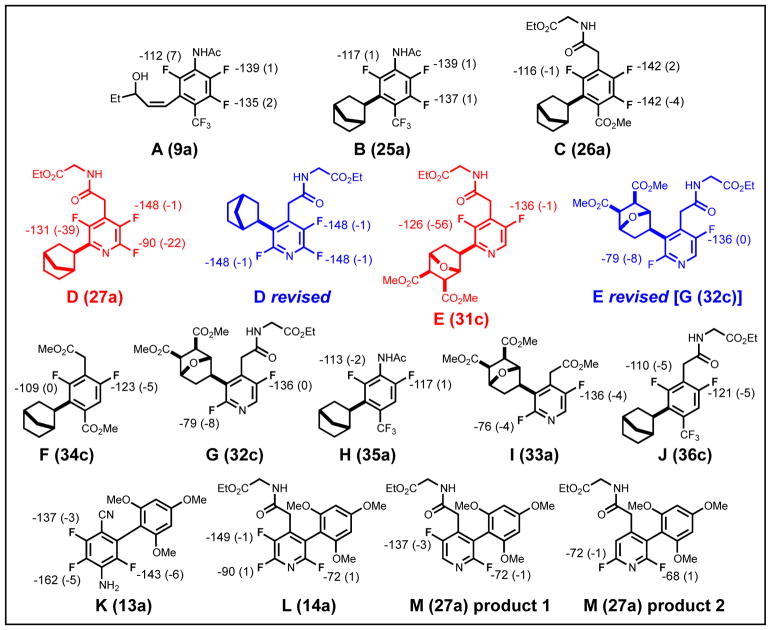
To put our scaling factors to the test, we applied them to several multifluorinated compounds synthesized in one of our labs (Chart 2). The original assignment of one structure was found to be in error on the basis of our predicted 19F shifts. The shifts for the corrected structure (Chart 2) are completely consistent with the experimental spectrum.
Chart 2.
Initial compounds explored, including a structure that was corrected by computations (highlighted in red). Computed shifts and error from experiment are shown for each aromatic fluorine.
After discovering this misassignment, a group of 13 substituted benzene and pyridine compounds with various substitution patterns (Chart 3) were examined to confirm, or correct, the published structures.7, 9, 12 Although many of the spectra match experiment, two of the structures had large errors (Chart 3, red). Based on the correction described above, revised structures were tested and these match the experimental values within the error expected for our scaling factors. These straightforward applications of computational 19F shift prediction highlight its utility in avoiding mistakes in assignments for highly fluorinated compounds.
Chart 3.
Fluorinated aromatic compounds for which assignments were not certain. Numbers in parenthases are numbering from the original paper. The calculated shifts and error from experimental shift are shown for each aromatic fluorine.
Conclusion
We have developed scaling factors aimed at fast and accurate prediction of 19F shifts for fluoroaromatics. In particular, the methods described here allow for the assignment of challenging structures bearing multiple fluorine atoms. The application of these scaling factors does not require advanced computations and, as a result, we hope that it will be applied broadly.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from the National Science Foundation (CHE-1565933, CHE-0957416 and CHE-030089 [via XSEDE]).
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website.
Energies for all computed structures, table with shifts and experimental solvents for all compounds used (PDF)
Coordinates for all computed structures (mol2)
References
- 1.Berger R, Resnati G, Metrangolo P, Weber E, Hulliger J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chemical Society Reviews. 2011;40(7):3496–3508. doi: 10.1039/c0cs00221f. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara T, O’Hagan D. Successful fluorine-containing herbicide agrochemicals. Journal of Fluorine Chemistry. 2014;167(Supplement C):16–29. [Google Scholar]
- 3.Theodoridis G. Chapter 4 Fluorine-Containing Agrochemicals: An Overview of Recent Developments. In: Tressaud A, editor. Advances in Fluorine Science. Vol. 2. Elsevier; 2006. pp. 121–175. [Google Scholar]
- 4.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chemical Society Reviews. 2008;37(2):320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- 5.Senaweera SM, Singh A, Weaver JD. Photocatalytic Hydrodefluorination: Facile Access to Partially Fluorinated Aromatics. Journal of the American Chemical Society. 2014;136(8):3002–3005. doi: 10.1021/ja500031m. [DOI] [PubMed] [Google Scholar]
- 6.Senaweera SM, Weaver JD. Selective Perfluoro- and Polyfluoroarylation of Meldrum’s Acid. The Journal of Organic Chemistry. 2014;79(21):10466–10476. doi: 10.1021/jo502075p. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Kubik JJ, Weaver JD. Photocatalytic C-F alkylation; facile access to multifluorinated arenes. Chemical Science. 2015;6(12):7206–7212. doi: 10.1039/c5sc03013g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora A, Weaver JD. Visible Light Photocatalysis for the Generation and Use of Reactive Azolyl and Polyfluoroaryl Intermediates. Accounts of Chemical Research. 2016;49(10):2273–2283. doi: 10.1021/acs.accounts.6b00259. [DOI] [PubMed] [Google Scholar]
- 9.Senaweera S, Weaver JD. Dual C–F, C–H Functionalization via Photocatalysis: Access to Multifluorinated Biaryls. Journal of the American Chemical Society. 2016;138(8):2520–2523. doi: 10.1021/jacs.5b13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cybulski MK, Nicholls JE, Lowe JP, Mahon MF, Whittlesey MK. Catalytic Hydrodefluorination of Fluoroarenes Using Ru(IMe4)2L2H2 (IMe4 = 1,3,4,5-Tetramethylimidazol-2-ylidene; L2 = (PPh3)2, dppe, dppp, dppm) Complexes. Organometallics. 2017;36(12):2308–2316. [Google Scholar]
- 11.Liu Y, Zhou Y, Zhao Y, Qu J. Synthesis of gem-Difluoroallylboronates via FeCl2-Catalyzed Boration/β-Fluorine Elimination of Trifluoromethyl Alkenes. Organic Letters. 2017;19(4):946–949. doi: 10.1021/acs.orglett.7b00168. [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Fennell CJ, Weaver JD. Photocatalyst size controls electron and energy transfer: selectable E/Z isomer synthesis via C-F alkenylation. Chemical Science. 2016;7(11):6796–6802. doi: 10.1039/c6sc02422j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolbier WR. Guide to Fluorine NMR for Organic Chemists. Wiley; 2009. [Google Scholar]
- 14.Muir M, Baker J. A simple calculational model for predicting the site for nucleophilic substitution in aromatic perfluorocarbons. Journal of Fluorine Chemistry. 2005;126(5):727–738. [Google Scholar]
- 15.Lodewyk MW, Siebert MR, Tantillo DJ. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chemical Reviews. 2012;112(3):1839–1862. doi: 10.1021/cr200106v. [DOI] [PubMed] [Google Scholar]
- 16.Grimblat N, Sarotti AM. Computational Chemistry to the Rescue: Modern Toolboxes for the Assignment of Complex Molecules by GIAO NMR Calculations. Chemistry – A European Journal. 2016;22(35):12246–12261. doi: 10.1002/chem.201601150. [DOI] [PubMed] [Google Scholar]
- 17.Di Micco S, Chini MG, Riccio R, Bifulco G. Quantum Mechanical Calculation of NMR Parameters in the Stereostructural Determination of Natural Products. European Journal of Organic Chemistry. 2010;2010(8):1411–1434. [Google Scholar]
- 18.Willoughby PH, Jansma MJ, Hoye TR. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nature Protocols. 2014;9:643. doi: 10.1038/nprot.2014.042. [DOI] [PubMed] [Google Scholar]
- 19.Jain R, Bally T, Rablen PR. Calculating Accurate Proton Chemical Shifts of Organic Molecules with Density Functional Methods and Modest Basis Sets. The Journal of Organic Chemistry. 2009;74(11):4017–4023. doi: 10.1021/jo900482q. [DOI] [PubMed] [Google Scholar]
- 20.Bally T, Rablen PR. Quantum-Chemical Simulation of 1H NMR Spectra. 2. Comparison of DFT-Based Procedures for Computing Proton–Proton Coupling Constants in Organic Molecules. The Journal of Organic Chemistry. 2011;76(12):4818–4830. doi: 10.1021/jo200513q. [DOI] [PubMed] [Google Scholar]
- 21.Fadeev DS, Chuikov IP, Mamatyuk VI. Non-empirical and DFT calculations of 19F and 13C chemical shifts in the NMR spectra of substituted pentafluorobenzenes. Journal of Structural Chemistry. 2013;54(1):180–185. [Google Scholar]
- 22.Ghiviriga I, Zhang L, Martinez H, Contreras RH, Tormena CF, Nodin L, Dolbier WR. 19F chemical shifts, coupling constants and conformational preferences in monosubstituted perfluoroparacyclophanes. Magnetic Resonance in Chemistry. 2011;49(3):93–105. doi: 10.1002/mrc.2713. [DOI] [PubMed] [Google Scholar]
- 23.Kasireddy C, Bann JG, Mitchell-Koch KR. Demystifying fluorine chemical shifts: electronic structure calculations address origins of seemingly anomalous 19F-NMR spectra of fluorohistidine isomers and analogues. Physical Chemistry Chemical Physics. 2015;17(45):30606–30612. doi: 10.1039/c5cp05502d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders LK, Oldfield E. Theoretical Investigation of 19F NMR Chemical Shielding Tensors in Fluorobenzenes. The Journal of Physical Chemistry A. 2001;105(34):8098–8104. [Google Scholar]
- 25.Shaghaghi H, Ebrahimi H, Tafazzoli M, Jalali-Heravi M. A survey of wave function effects on theoretical calculation of gas phase 19F NMR chemical shifts using factorial design. Journal of Fluorine Chemistry. 2010;131(1):47–52. [Google Scholar]
- 26.Sternberg U, Klipfel M, Grage SL, Witter R, Ulrich AS. Calculation of fluorine chemical shift tensors for the interpretation of oriented 19F-NMR spectra of gramicidin A in membranes. Physical Chemistry Chemical Physics. 2009;11(32):7048–7060. doi: 10.1039/b908236k. [DOI] [PubMed] [Google Scholar]
- 27.Hogben MG, Graham WAG. Chemical shifts and coupling constants in pentafluorophenyl derivatives. I. Correlations of chemical shifts, coupling constants, and .pi.-electronic interactions. Journal of the American Chemical Society. 1969;91(2):283–291. [Google Scholar]
- 28.Saielli G, Bini R, Bagno A. Computational 19F NMR. 2. Organic compounds. RSC Advances. 2014;4(78):41605–41611. [Google Scholar]
- 29.He K-H, Tan F-F, Zhou C-Z, Zhou G-J, Yang X-L, Li Y. Acceptorless Dehydrogenation of N-Heterocycles by Merging Visible-Light Photoredox Catalysis and Cobalt Catalysis. Angewandte Chemie International Edition. 2017;56(11):3080–3084. doi: 10.1002/anie.201612486. [DOI] [PubMed] [Google Scholar]
- 30.Yuan X, Yao J-F, Tang Z-Y. Decarboxylative Fluorination of Electron-Rich Heteroaromatic Carboxylic Acids with Selectfluor. Organic Letters. 2017;19(6):1410–1413. doi: 10.1021/acs.orglett.7b00335. [DOI] [PubMed] [Google Scholar]
- 31.Boden N, Emsley JW, Feeney J, Sutcliffe LH. The effects of π-electron distribution and intramolecular electric fields on the 19F N.M.R. shielding in substituted perfluorobenzenes. Molecular Physics. 1964;8(2):133–149. [Google Scholar]
- 32.Plevey RG, Rendell RW, Tatlow JC. Fluorinations with complex metal fluorides. Part 6 [1] fluorination of yridine and related compounds with caesium tetrafluorocobaltate(III) [2] Journal of Fluorine Chemistry. 1982;21(2):159–169. [Google Scholar]
- 33.Coe PL, Holton AG, Tatlow JC. Fluorinations with potassium tetrafluorocobaltate(III) Part VII. Further investigations on the fluorination of pyridine. Journal of Fluorine Chemistry. 1982;21(2):171–189. [Google Scholar]
- 34.Gribble GW, Keavy DJ, Olson ER, Rae ID, Staffa A, Herr TE, Ferraro MB, Contreras RH. Fluorine deshielding in the proximity of a methyl group. An experimental and theoretical study. Magnetic Resonance in Chemistry. 1991;29(5):422–432. [Google Scholar]
- 35.Burdon J, Chivers GE, Tatlow JC. Highly fluorinated heterocycles. Part III. The preparation and reactions of some polyfluorofurans. Journal of the Chemical Society C: Organic. 1970;(16):2146–2151. [Google Scholar]
- 36.Burdon J, Campbell JG, Parsons IW, Tatlow JC. Highly fluorinated heterocycles. Part V. The preparation and some reactions of tetrafluorothiophen and some polyfluorothiophens. Journal of the Chemical Society C: Organic. 1971;(0):352–355. [Google Scholar]
- 37.Baasner B, Klauke E. A new route to the synthesis of 5-fluorouracil. Journal of Fluorine Chemistry. 1989;45(3):417–430. [Google Scholar]
- 38.Chambers DR, Hall WC, Hutchinson J, Millar WR. Polyhalogenated heterocyclic compounds. Part 42.1 Fluorinated nitrogen heterocycles with unusual substitution patterns. Journal of the Chemical Society, Perkin Transactions 1. 1998;(10):1705–1714. [Google Scholar]
- 39.Schlosser M, Bobbio C, Rausis T. Regiochemically Flexible Substitutions of Di-, Tri-, and Tetrahalopyridines: The Trialkylsilyl Trick. The Journal of Organic Chemistry. 2005;70(7):2494–2502. doi: 10.1021/jo047962z. [DOI] [PubMed] [Google Scholar]
- 40.Bobbio C, Rausis T, Schlosser M. Removal of Fluorine from and Introduction of Fluorine into Polyhalopyridines: An Exercise in Nucleophilic Hetarenic Substitution. Chemistry – A European Journal. 2005;11(6):1903–1910. doi: 10.1002/chem.200400837. [DOI] [PubMed] [Google Scholar]
- 41.Božilović J, Bats JW, Engels JW. Synthesis and structure of fluoroindole nucleosides. Canadian Journal of Chemistry. 2007;85(4):283–292. [Google Scholar]
- 42.Lemal DM, Akashi M, Lou Y, Kumar V. Tetrafluorothiophene S,S-Dioxide: A Perfluorinated Building Block. The Journal of Organic Chemistry. 2013;78(24):12330–12337. doi: 10.1021/jo402373x. [DOI] [PubMed] [Google Scholar]
- 43.Becke AD. A new mixing of Hartree–Fock and local density-functional theories. The Journal of Chemical Physics. 1993;98(2):1372–1377. [Google Scholar]
- 44.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics. 1993;98(7):5648–5652. [Google Scholar]
- 45.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B. 1988;37(2):785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 46.Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. The Journal of Physical Chemistry. 1994;98(45):11623–11627. [Google Scholar]
- 47.Tirado-Rives J, Jorgensen WL. Performance of B3LYP Density Functional Methods for a Large Set of Organic Molecules. J Chem Theory Comput. 2008;4(2):297–306. doi: 10.1021/ct700248k. [DOI] [PubMed] [Google Scholar]
- 48.London F. Théorie quantique des courants interatomiques dans les combinaisons aromatiques. J Phys Radium. 1937;8(10):397–409. [Google Scholar]
- 49.McWeeny R. Perturbation Theory for the Fock-Dirac Density Matrix. Physical Review. 1962;126(3):1028–1034. [Google Scholar]
- 50.Ditchfield R. Self-consistent perturbation theory of diamagnetism. Molecular Physics. 1974;27(4):789–807. [Google Scholar]
- 51.Wolinski K, Hinton JF, Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. Journal of the American Chemical Society. 1990;112(23):8251–8260. [Google Scholar]
- 52.Cheeseman JR, Trucks GW, Keith TA, Frisch MJ. A comparison of models for calculating nuclear magnetic resonance shielding tensors. The Journal of Chemical Physics. 1996;104(14):5497–5509. [Google Scholar]
- 53.Bagno A, Saielli G. Addressing the stereochemistry of complex organic molecules by density functional theory-NMR. Wiley Interdisciplinary Reviews: Computational Molecular Science. 2015;5(2):228–240. [Google Scholar]
- 54.de Albuquerque AC, Ribeiro DJ, de Amorim MB. Structural determination of complex natural products by quantum mechanical calculations of (13)C NMR chemical shifts: development of a parameterized protocol for terpenes. J Mol Model. 2016;22(8):183. doi: 10.1007/s00894-016-3045-6. [DOI] [PubMed] [Google Scholar]
- 55.Marenich AV, Cramer CJ, Truhlar DG. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. The Journal of Physical Chemistry B. 2009;113(18):6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 56.Spartan’10. Wavefunction, Ind; Irvine, CA, USA: 2010. [Google Scholar]
- 57.Gaussian 09, Revision D. 01. Gaussian, Inc; Wallingford, CT, USA: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.