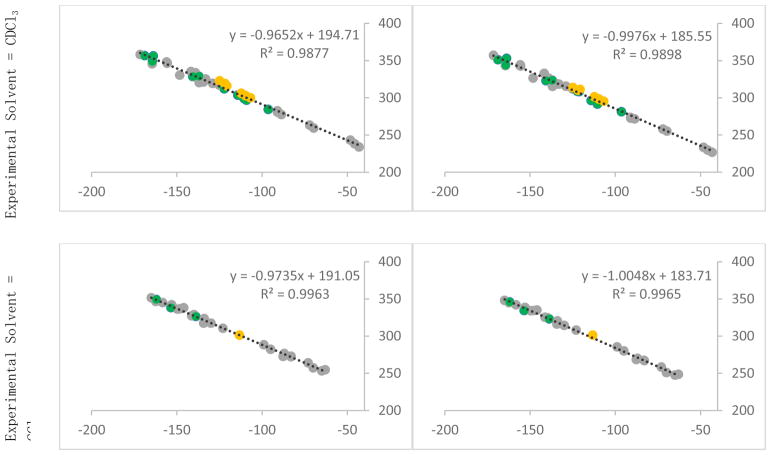
Figure 2.
Experimental and computed shifts for the training set used for optomization and NMR calculation at B3LYP/6-31+G(d,p) – structures taken in chloroform (top) and calculated in gas (left) and chloroform (right), structures taken in carbon tetrachloride (bottom). Mono-cyclic aromatic compounds (group A) are shown in green, non-heterocyclic compounds (group B) are shown in grey, and bicyclic-heterocyclic compounds (group C) are shown in yellow.