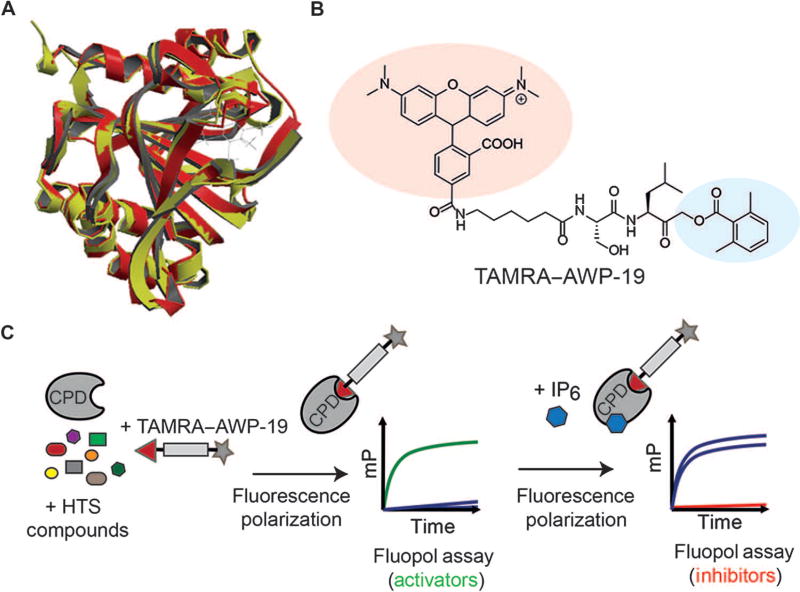
Fig. 1. Screening for small-molecule activators and inhibitors of the CPD of TcdB.
(A) Superposition of the crystal structures of TcdB (red) and TcdA (yellow) CPD domains with an IP6 allosteric activator bound. (B) Structure of the activity-based probe TAMRA–AWP-19 used for the high-throughput screen (HTS). The fluorophore is shaded in red, and the acyloxymethyl ketone electrophile specific for cysteine proteases is shown in blue. (C) Schematic of the fluorescence polarization (fluopol)–activity-based probe screen workflow. Compounds were added to the purified CPD domain from TcdB followed by addition of TAMRA–AWP-19 probe. The unbound probe produced low fluorescence polarization [millipolarization (mP)], which increased upon binding to the activated CPD. This change in millipolarization was measured on a plate reader and was used to assess the ability of a compound to either directly stimulate or inhibit (upon IP6 addition) CPD activation.