Fig. 4. S100B inhibits surface-catalyzed secondary nucleation of Aβ42 oligomers.
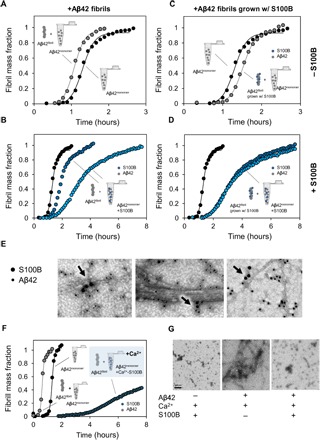
(A) Aggregation kinetics of 5 μM Aβ42 (black) seeded by the addition of 0.1 μM preformed Aβ42 fibrils (gray). (B) Aggregation kinetics of 5 μM Aβ42 (black) seeded by the addition of 0.1 μM preformed Aβ42 fibrils in the presence of 50 μM S100B (dark blue) and 150 μM S100B (light blue) in solution. (C) Aggregation kinetics of 5 μM Aβ42 (black) seeded by the addition of 0.1 μM preformed Aβ42 fibrils grown with S100B (gray). (D) Aggregation kinetics of 5 μM Aβ42 (black) seeded by the addition of 0.1 μM preformed Aβ42 fibrils grown with S100B in the presence of 50 μM S100B (dark blue) and 150 μM S100B (light blue) in solution. (E) TEM images with a nanogold-conjugated secondary antibody against S100B (15 nm) and Aβ42 (10 nm), showing binding of S100B to fibrils and oligomers. (F) Aggregation kinetics of 10 μM Aβ42 with and without the addition of 0.1 μM preformed Aβ42 fibrils in the presence of 50 μM Ca2+-S100B (dark blue) in solution. (G) TEM images of end points of ThT aggregation kinetics of 150 μM S100B, 10 μM Aβ42, and 10 μM Aβ42 + 150 μM S100B, with 1.1 mM CaCl2.
