Fig. 6. S100B delays Aβ42 aggregation by suppressing primary and secondary nucleation.
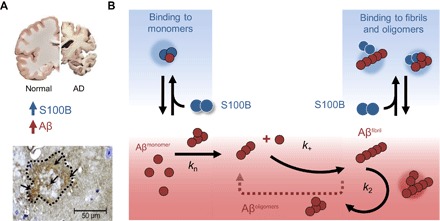
(A) In the AD brain, protein aggregation and exacerbated inflammation are well-established disease features, with elevated levels of Aβ42 and S100B. In AD animal models such as the 5XFAD mice, amyloid plaques (dotted contour) have intense staining for extracellular S100B (arrows) (see also fig. S7). (B) Extracellular S100B and Aβ42 engage in regulatory interactions, which are depicted in the scheme which summarizes the finding that S100B inhibits Aβ42 fibril formation mainly by affecting the lag phase and secondary nucleation, through interactions with monomeric and fibrillar Aβ42, suggesting a potential role of S100B as novel extracellular chaperone suppressing proteotoxicity in AD.
