Fig. 3. Permeability trends of lipid bilayers, BCP bilayers, and conventional desalination membranes.
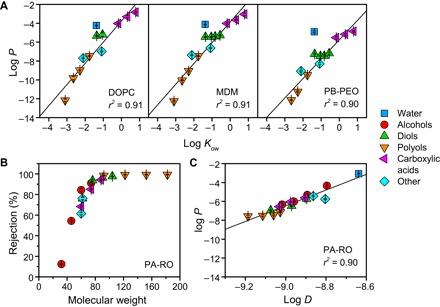
(A) Solubility-based permeability of fluid-like lipid and amphiphilic BCP bilayers. Water and solute permeabilities P (in m/s) were measured at 25°C (n = 3) and are compared with the octanol-water partition coefficient Kow, a commonly used measure of solute hydrophobicity. Regression lines consider all species except for water because of its anomalously rapid permeation stemming from its small size (21, 51). The strong correlation between solute permeability and hydrophobicity matches and extends Overton’s rule, which was originally formulated for lipid bilayers (21). (B) Solute rejection, defined as 1 − Cpermeate/Cfeed, for a commercial PA-RO membrane (SW30XLE, Dow), measured at 15.5 bar and 25°C (n = 6). Rejection is size-based, with a molecular weight cutoff of ~80 Da, which leads to (C) a strong dependence of permeability (in m/s) on the solute diffusivity in water D (in m2/s), which decreases with increasing solute size.
