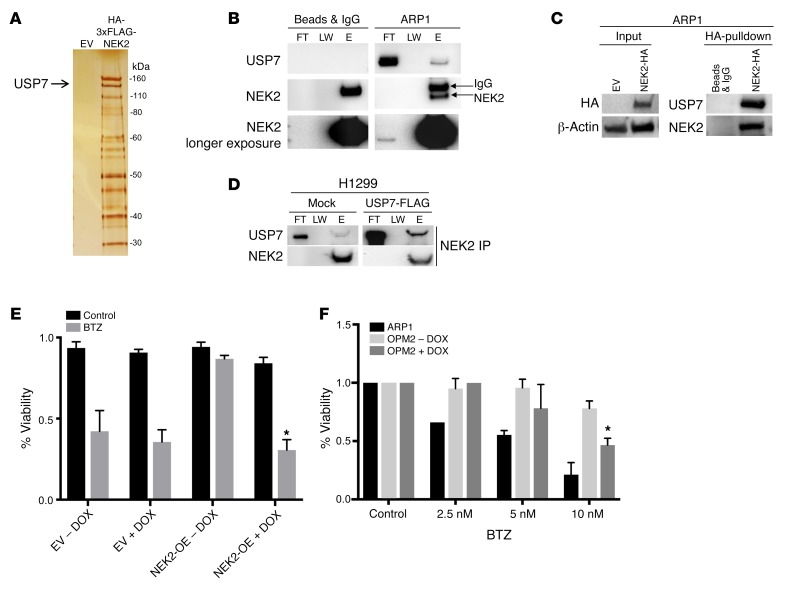
Figure 1. USP7 interacts with NEK2.
(A) HEK293T cells were transfected with either empty vector (EV) or HA-FLAG(3×)-NEK2. Proteins binding to NEK2 were pulled down by tandem HA and FLAG antibodies and stained with silver prior to mass spectrometry. (B) ARP1 myeloma cells were lysed and NEK2 was immunoprecipitated using NEK2 antibodies. Western blots were probed with NEK2 and USP7 antibodies. FT, LW, and E represent flow through, last wash, and elution of the immunoprecipitation, respectively. (C) ARP1 myeloma cells were transduced with NEK2-HA plasmids. Transduced cells were lysed and NEK2 was immunoprecipitated using HA antibodies. Western blots were probed using NEK2 and USP7 antibodies. (D) H1299 cells were transfected with mock or USP7-FLAG overexpression vector. Endogenous NEK2 was immunoprecipitated and Western blots were analyzed using NEK2 and USP7 antibodies. (E) ARP1 myeloma cells transduced with EV + USP7-shRNA or NEK2-OE + USP7-shRNA were treated with doxycycline (DOX) or vehicle to suppress USP7 expression. After 72 hours, cells were treated with bortezomib (BTZ; 5 nM) for a further 24 hours and cell viability was measured using trypan blue stain. (F) OPM2 cells transduced with NEK2-shRNA were treated with DOX or vehicle to suppress NEK2 expression. ARP1 cells or OPM2 cells with or without silencing of NEK2 were treated with BTZ (2.5, 5, and 10 nM) for a further 24 hours and cell viability was measured using trypan blue stain. Viability experiments were performed in triplicate and a Student’s t test was performed and showed the significance at 10 nM with or without silencing of NEK2. *P < 0.05.