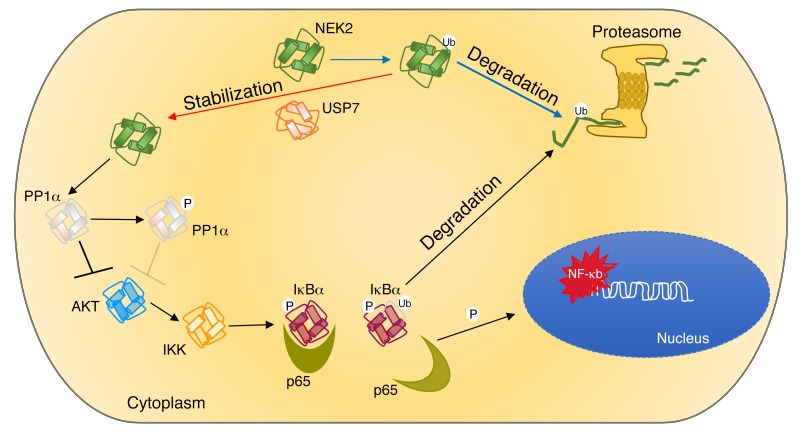
Figure 11. Working model of the interaction between NEK2 and USP7.
USP7 binds to and stabilizes NEK2 by deubiquitination, allowing it to accumulate in myeloma cells. Accumulated NEK2 binds to and phosphorylates PP1α, resulting in loss its AKT-suppressing activity. Active AKT triggers the canonical NF-κB pathway by phosphorylating IKK, with subsequent phosphorylation and degradation of IκBα. p65 released from the complex with IκBα translocates into the nucleus, where it activates its target genes leading to drug resistance in myeloma. Ub, ubiquitin.