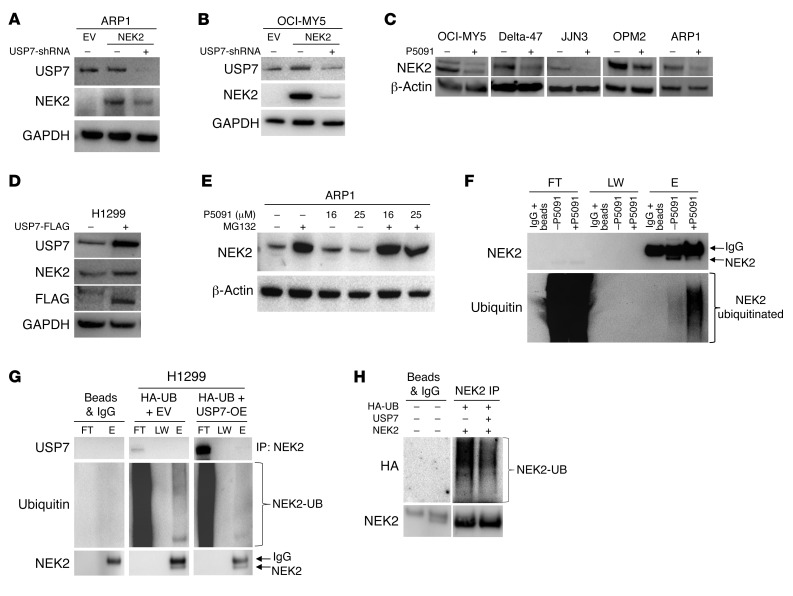
Figure 2. USP7 prevents ubiquitination of NEK2 protein.
(A and B) Knockdown of USP7 decreases NEK2 protein. ARP1 (A) and OCI-MY5 (B) myeloma cells were transfected with EV, NEK2, or NEK2 + USP7-shRNA. After 72-hour induction with doxycycline, cells were lysed. NEK2 and USP7 protein levels were analyzed by Western blot. (C) OCI-MY5, Delta-47, JJN3, OPM2, and ARP1 myeloma cell lines were treated with 16 μM P5091 for 24 hours. Cells were lysed and NEK2 levels analyzed by Western blot. (D) H1299 cells were transfected with mock or USP7-FLAG–overexpressing vectors, lysed, and NEK2 and USP7 levels were determined by Western blot. (E) ARP1 myeloma cells were treated with the proteasome inhibitor MG132 (10 μM) alone for 30 minutes or in combination with P5091 (16 and 25 μM) for an additional 5 hours. Cells were lysed and NEK2 levels were analyzed by Western blot. (F) OPM2 cells were treated with or without P5091 (25 μM for 2 hours) and protein was extracted with lysis buffer supplemented with NEM. Endogenous NEK2 was immunoprecipitated and analyzed by Western blot using NEK2 and ubiquitin antibodies. FT, LW, and E represent flow through, last wash, and elution of the immunoprecipitation, respectively. (G) H1299 cells were transfected with EV and HA-ubiquitin (HA-UB) or FLAG-USP7 and HA-UB. Cells were lysed and endogenous NEK2 was immunoprecipitated (IP) by NEK2 antibodies and ubiquitination levels were analyzed by Western blot. The higher-molecular-weight band is nonspecific IgG. (H) H1299 cells were transfected with NEK2-OE, HA-UB, and FLAG-USP7 or NEK2-OE and HA-UB. Cells were lysed and total NEK2 protein, including both endogenous and exogenous, was immunoprecipitated (IP) by anti-NEK2 antibodies and ubiquitination levels were analyzed using HA antibodies by Western blot.