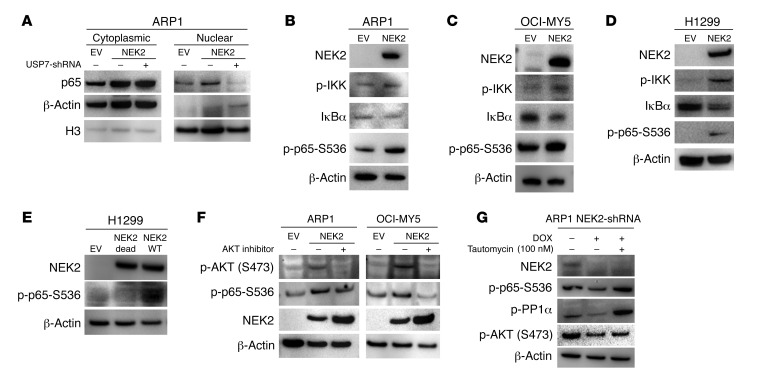
Figure 5. NEK2 activates NF-κB signaling via PP1α/AKT.
(A) USP7 was knocked down in ARP1 cells transduced with NEK2-OE after 72 hours induction with doxycycline (DOX). Nuclear and cytosolic fractionations were carried out. p65 levels were analyzed between EV and NEK2-OE with or without USP7 shRNA by Western blot. β-Actin and histone H3 (H3) were used as cytosolic and nuclear markers, respectively. (B–D) EV and NEK2-OE ARP1, OCI-MY5, and H1299 cells were lysed. NEK2, p65-S536 phosphorylation, IKK phosphorylation, and IκBα were analyzed by Western blot. (E) H1299 cells transiently transfected with EV or NEK2-OE (WT) or NEK2-K37R mutant (NEK2-Dead) were lysed, and NEK2 and p65-S536 phosphorylation was analyzed by Western blot. (F) ARP1 and OCI-MY5 cells transfected with EV or NEK2-OE were treated with vehicle or MK-2206 2HCl, an AKT inhibitor, for 30 minutes and then cells were lysed. p65-S536 phosphorylation was analyzed by Western blot. (G) NEK2-shRNA ARP1 cells were induced with DOX for 48 hours and then treated with tautomycin, a PP1α inhibitor, for another 24 hours. NEK2, p-p65-S536, p-PP1α, and p-AKT were analyzed by Western blot.