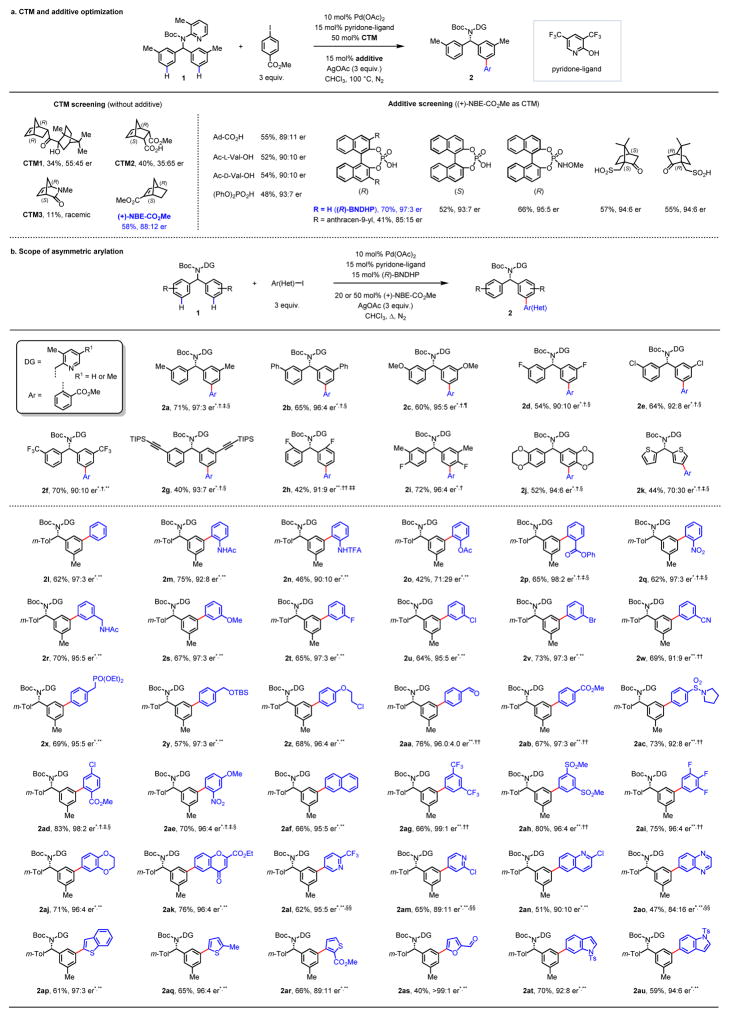
Figure 2. Enantioselective meta-C–H arylation of diarylmethylamines.
a, CTM and additive optimization. Reaction conditions: 10 mol% Pd(OAc)2, 15 mol% pyridone-ligand, 50 mol% (+)-NBE-CO2Me, 15 mol% additive, 3 equiv. methyl 4-iodobenzoate, 3 equiv. AgOAc, CHCl3, 100 °C. For each entry number (in bold), data are reported as NMR yield. b, Scope of asymmetric arylation. Reaction conditions: 10 mol% Pd(OAc)2, 15 mol% pyridone-ligand, 20 mol% (+)-NBE-CO2Me, 15 mol% (R)-BNDHP, 3 equiv. Ar–I, 3 equiv. AgOAc, CHCl3, 100 °C. *R1 = Me. †15 mol% (PhO)2PO2H. ‡1.5 equiv. Ar–I, 2 equiv. AgOAc. §80 °C. ¶60 °C. **50 mol% (+)-NBE-CO2Me. ††R1 = H. ‡‡20 mol% Pd(OAc)2, 30 mol% pyridone-ligand, 30 mol% (R)-BNDHP. §§15 mol% Pd(OAc)2, 23 mol% pyridone-ligand. For each entry number (in bold), data are reported as isolated yield. The absolute configuration of 2ah was determined by X-ray crystallography. DG, directing group; Ar, aryl group; m-Tol, meta-tolyl group. Reducing the catalyst loading to 5 mol% produced comparable results for substrate 1a′ (see Table S6 in Supplementary Information).