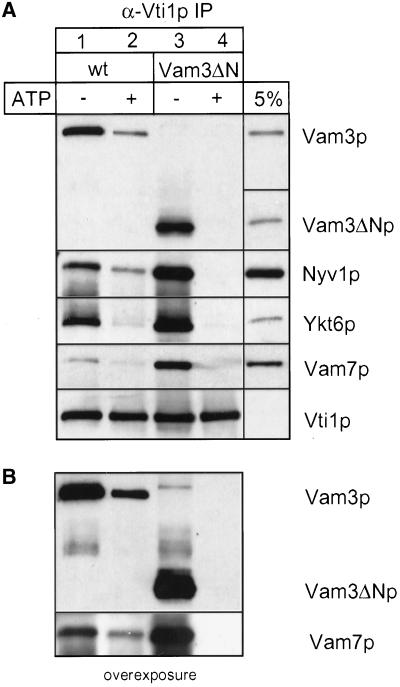
Figure 4.
Vam3ΔN assembles into cis-SNARE complexes. Wild-type or mutant vacuoles (60 μg each) were incubated in reaction buffer with cytosol, with or without ATP at 26°C. After 10 min the vacuoles were collected by centrifugation. Solubilization and immunoprecipitation was performed as described in MATERIALS AND METHODS. Immunoprecipitation was done with a polyclonal anti-Vti1p antiserum covalently coupled to Protein A–Sepharose beads (Ungermann et al., 1999). Proteins were eluted with 0.1 M glycine, pH 2.6, precipitated with TCA, separated on 15% SDS-polyacrylamide gels, and detected with the indicated antibodies (A). A fraction of the wild-type detergent extract (5%; for Vam3p, Vam7p, Vti1p, and Ykt6p) as well as a fraction of the mutant extract (Vam3ΔN) was precipitated and loaded as a controls. The amount of coprecipitated SNAREs was quantified densitometrically (NIH image 1.6): wild-type − ATP, 100 ± 16; wild-type + ATP, 46 ± 10; Vam3ΔN − ATP, 225 ± 14; Vam3ΔN + ATP, 24 ± 4 (mean density after background subtraction in arbitrary units ± SEM, n = 4). (B) An overexposure of the immunoblots decorated with anti-Vam3p and anti-Vam7p.