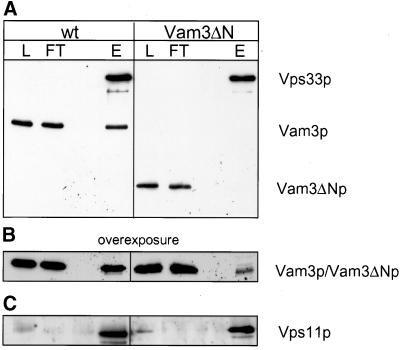
Figure 8.
Efficient recruitment of HOPS/Vps33p to Vam3p depends on the N-terminal domain. Vacuoles (300 μg) prepared from BJ VPS33:: ProtA or BJ VAM3ΔN VPS33:: ProtA strains, were solubilized, and Protein A–tagged Vps33p was purified as described in MATERIALS AND METHODS. Fractions of the Protein-A-purification were precipitated with TCA, separated on a 15% SDS gel and blotted onto nitrocellulose. The Western blot probed with antibodies against Vam3p (A and B) or Vps11 (C). L = 1% of total protein loaded on the column; FT = 1% of the flow-through; E = 30% of the eluate. The left panel shows the isolation from vacuoles with full length Vam3p and the right panel the isolation from Vam3ΔN vacuoles (The high-molecular-weight band is Vps33p, which was detected by the secondary antibody because of the Protein A–tag).